First termed in 1942 by A.H. Coons, FITC is a unique fluorescein molecule having an isothiocyanate reactive group (-N=C=S), which replaced the hydrogen atom located at the base of its structure. In this article, we’ll discuss FITC labeling techniques and provide tips and tricks for maximizing yield.
Fluorescein isothiocyanate (FITC) has been extensively used for attaching a fluorescent moiety to proteins, antibodies, and lectins through an amine group. The isothiocyanate functional group reacts with primary amines of proteins at lysine residues and at the protein’s amino terminus.
What is FITC Labeling ?
FITC labeling involves conjugation of Fluorescein isothiocyanate to a biomolecule.
Fluorescein isothiocyanate (FITC) labeling is a common technique with a wide range of applications because it reacts quickly with amines and due to its high quantum efficacy. Due to its high molecular absorptivity, using FITC labels is preferred over conventional colorimetric labels and radio labels because fluorophores like FITC are bright, easier to work with, and don’t require special waste handling. Proteins, substrates, peptide hormones, and antibodies labeled by FITC can be used as probes in flow cytometry, enzyme kinetics, and immunocytochemistry, as well as in the detection of receptors on the surface of the target cells.
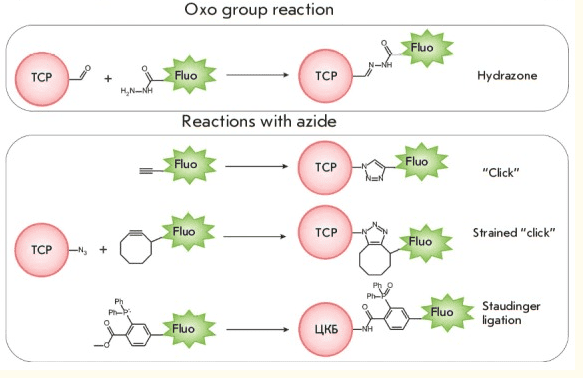
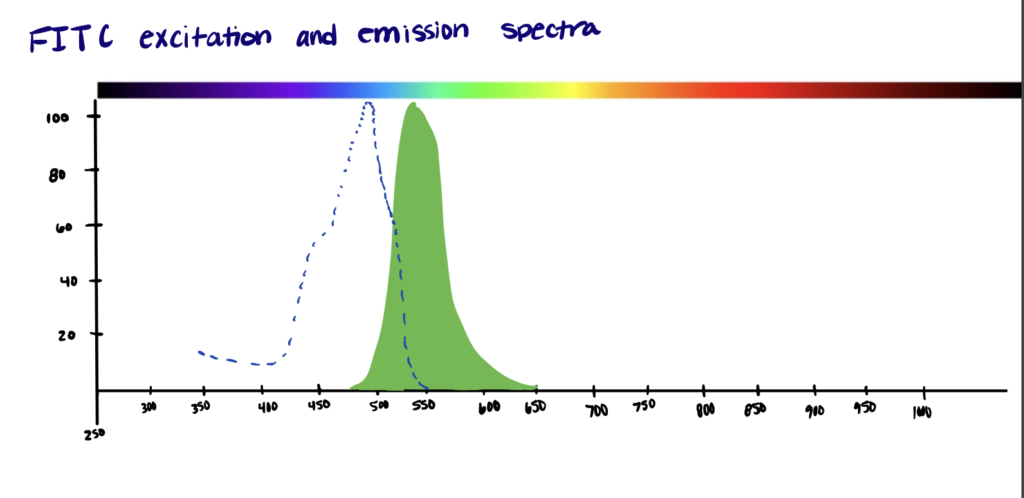
The excitation and emission wavelengths of FITC are 494nm and 518 nm, respectively, which gives it a green color. Originally, FITC was reported to have two isomers – fluorescein 5-isothiocyanate and fluorescein 6-isothiocyanate. A product of both isomers is also known to exist (fluorescein 5(6)-isothiocyanate).
However, attaching the fluorescent label to proteins requires the use of pure and highly concentrated proteins. Any amine-containing molecules in the protein buffer can compete with the reaction between FITC and the protein’s amines. Furthermore, because of its hydrophobic nature, attaching too many FITC molecules to a protein may cause aggregation or precipitation at high protein concentrations, which causes the experiment to fail. On the other hand, owing to the high molar absorptivity, the FITC labeling technique is preferred over other conventional labeling methods.
Amine Labeling
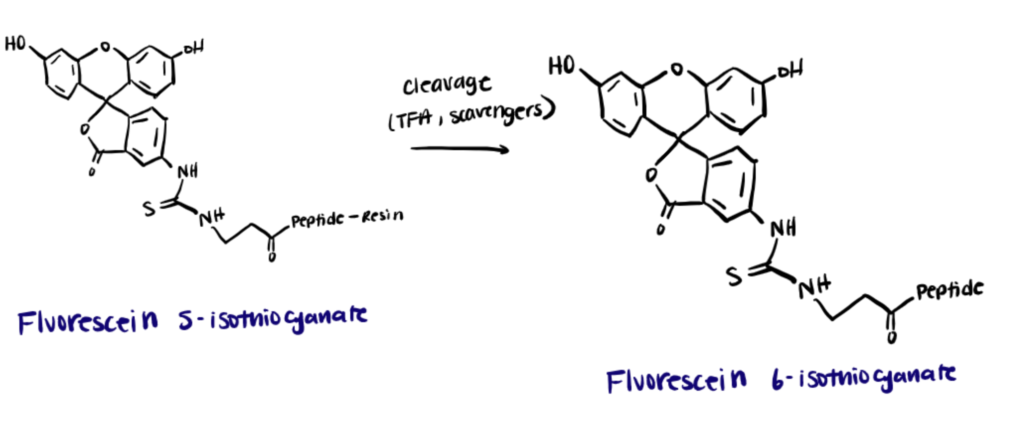
Isothiocyanates react with amines to form thioureas. While the thiourea is stable, it has been found that concentrated ammonia can cause a FITC conjugate to convert into a guanidine in the presence of concentrated ammonia (source: Thermo Fisher). Although there are several available amine-reactive fluorophores, fluorescein isothiocyanate (FITC) is the most commonly used for the preparation of fluorescent bioconjugates. (Chaganthi et al.)
Azide-FITC Reaction
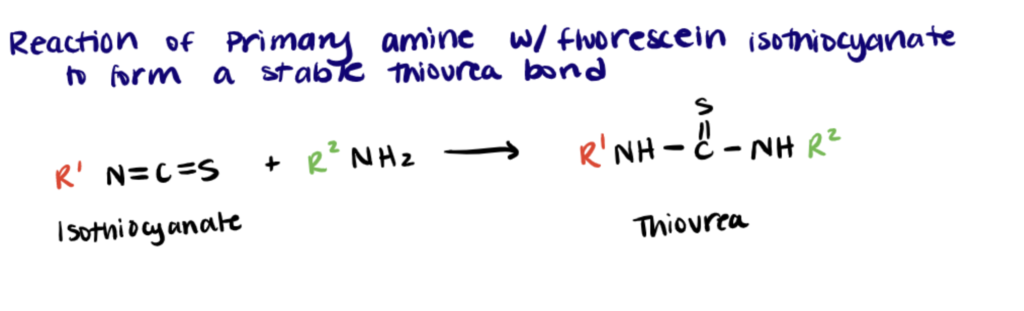
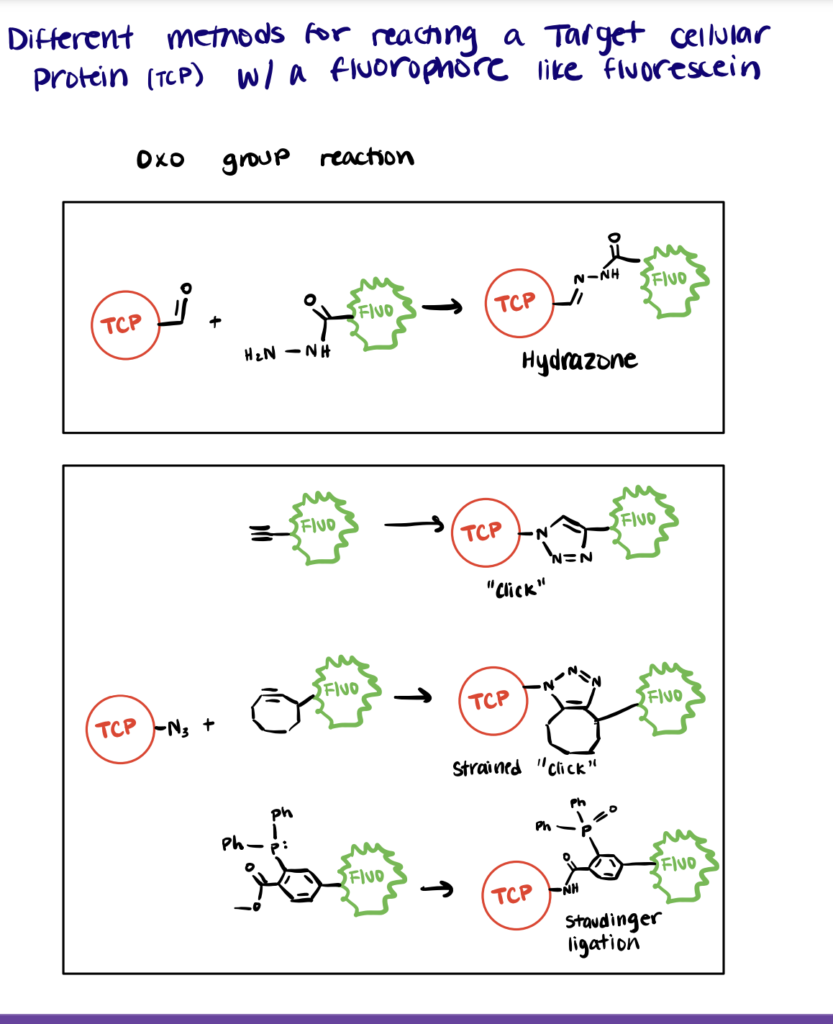
Azides do not directly react with isothiocyanates. In order to react an Azide with FITC, the FITC must first be linked to an alkyne.
However, fluorescein derivatives with alkynes can be reacted with target cellular proteins (TCPs) containing azides using copper-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted alkyne azide cycloaddition (SPAAC). To execute these strategies, first react cysteines or lysines on the TCP with an azide containing polymer. Then, react the azido-protein with a fluorescein-DBCO (dibenzocyclooctyne) or propargyl-fluorescein (source: Acta Naturae).
In many cases, the generation of Cu(I) for CuAAC will denature proteins, so SPAAC has become a more popular method for in situ Azide-FITC reactions.
FITC Protein Labeling Protocol
Utilizing FITC to attach fluorescent molecules to proteins is a commonly utilized strategy and is relatively easy to carry out. Below you’ll find a brief FITC protein labeling protocol, for more details take a look at this document.
To label proteins with FITC, first dissolve the protein in carbonate buffer, then incubate with FITC solution for 8 hours, and finally quench and separate the unconjugated FITC.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Step 1. Prepare FITC and Protein Solutions
First, prepare a protein solution containing 2 mg/ml of protein in 0.1M sodium carbonate buffer at ph 9. Note: Avoid using stored sodium carbonate/bicarbonate buffer for more than a week since the pH of the buffer fluctuates. The protein that is to be conjugated must be pure and free of contamination, and should not be prepared in buffer solutions containing amines or azides to avoid competing with the labeling reaction.
Then, use anhydrous DMSO to dissolve FITC at 1 mg/ml. Do not use stored solutions of FITC in DMSO; prepare fresh on the day of the reaction.
Step 2. Incubate Protein with FITC
Slowly and gently add 50 ul of FITC solution to each ml of protein solution in 5 ul intervals while stirring the mixture.
Incubate the solutions in the dark at 4 °C for 8 hours.
Step 3. Quench the Reaction with Excess Amines
Quench the reaction mixture with NH4Cl by adding enough to make a final concentration of 50 mM. Then incubate the reaction at 4°C for 2 hours.
To remove the unconjugated FITC, utilize dialysis or column chromatography. FITC is much smaller than any protein, so it is easy to separate based on size.
FITC labeling and FITC Staining
So far we have discussed a FITC labeling of proteins. Similar techniques can be applied to antibodies and any other amine-containing compounds.
FITC labeled molecules can also be used as a stain in cell analysis. Previously Chopra et al. have utilized FITC-labeled MHC-II antibodies to stain mouse bone marrow-derived dendritic cells for flow cytometry. Additionally, this kit from Abcam is an Annexin V-FITC apoptosis stain kit which detects apoptosis by staining phosphatidyl serine and tracking how many molecules have translocated to the outside of the cell membrane. Millipore also sells a FITC staining kit for identifying cellular substructures.
Applications of FITC labeling
Some applications of FITC labeling include flow cytometry, imaging receptor-mediated signaling, labeling antibodies for staining and other applications, as a pH indicator, apoptosis detection, and nucleotide labeling.
Flow Cytometry
Coulter established a process in 1945 that allows the measurement of cell sizes and counting events through conductance variation. In this method, FITC-labeled cells go through a thin capillary one-by-one and upon laser excitation, the extinction number determines the cell number. Though this method faces many challenges, Coulter accurately calculated volumes of cells suspended in a saline solution based on electrical resistance deviation – ie. both cells and sheath fluids are ionic solutions and cells coated in lipid membranes are bad conductors in comparison to the saline suspension. The Coulter cell counters later evolved into cell analyzers and were promptly embraced by several clinical laboratories and other industries for blood cell counting.
Receptor-Mediated Targeting
Many cells, such as cancers, overexpress certain receptors such as the folate receptor. Receptor-mediated targeting involves using antibodies or receptor substrates (ex: Folic acid) to target drugs to these cells. By utilizing this method, drugs can be enriched in the diseased tissue. You can learn more about bioconjugation and cellular uptake in our related article.
FITC is commonly used to label the drug carrier to track that it is entering target cells in vitro and in vivo. For example, FITC chitosan-based nanocarriers have been utilized to target several distinct tumor cells which overexpress target receptors on their outer surface. (Caprifico et al.)
Fluorescent Probe (Labeling antibodies)
Takai et al. discovered a method for antigen detection inside tissue cells in which fluorescein-labeled antibodies were utilized as a histochemical stain. The antibody molecule can be conjugated with chemical compounds without disrupting its ability to react with its corresponding antigen. Since then, this technique has been applied to demonstrate viral antigens of rickets and mumps inside infected cells(Sattar et al.). It was also used in research which studied the effect of pneumococcal capsular carbohydrate inserted within mouse cells. (Canaan-Haden et al.)
An alternative method for labeling antibodies involves the bioconjugation of metal complexes or labeling with quantum dots. Learn more in our related articles.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
pH Indicator
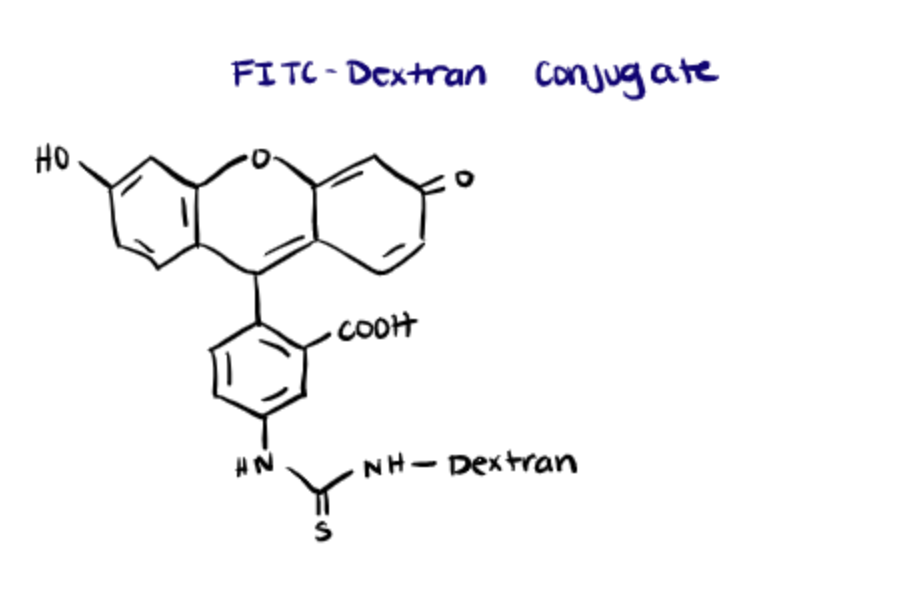
Fluorescent dyes like FITC possess the ability to change color in response to alterations in the pH. FITC can change colors when it is a fluorescent dye, alone, or a fluorescent macromolecule such as FITC-conjugated dextran (tdlabs). This principle can be employed to measure the pH inside cells and to track alterations in the pH due to physiological processes such as cell proliferation, ion transport, muscle contraction, and apoptosis.
The primary benefit of using macromolecule complexes of FITC such as FITC-dextran is that the molecules can be used to probe cellular compartments. In this article, Raman et al. found that fluorescein-labeled heparin derivatives internalized into different cellular compartments depending on sulfation patterns.
Using fluorescein based pH-indicators also provides a better spatial sample than microelectrode techniques since fluorescent dyes can enter all cellular compartments at once whereas microelectrodes can only be used to analyze one portion of a singular cellular component at a time. Another big benefit of the fluorescent-based indicators and probes is that they do not bind intracellular proteins after they have been conjugated.
Apoptosis detection
Fluorescein isothiocyanate (FITC) plays an important role in the detection of
apoptosis. The main methods include direct DNA labeling with fluorescein-tagged nucleotides (used in the TUNEL assay) and conjugation of fluorescein with annexin (Labome). Another method involves the detection of caspase activity via fluorescent-tagged Z-VAD-FMK (a caspase inhibitor).
Annexin V is present in the outermost layer of the cell membrane during apoptosis and binds with phosphatidylserine. Using a microscope or flow cytochemistry, the apoptotic or dead cells can easily be detected by fluorescein-labeled annexin. Annexin V-FITC apoptosis detection kits can be used to examine the glucose control in murine blood and the origin of prostate cancer in humans. Fluorescein conjugated annexin V manufactured by BD Biosciences is being used in immunomicroscopy as well as in flow cytometry to investigate the JAK2 mutational effects on hematopoietic stem cells.
Nucleotide Labeling
Apart from the TUNEL assay which makes use of the FITC labeling, FITC-tagged nucleotides are widely being used in the cell proliferation assays, for instance, in flow cytometry as BrdU-conjugates, or employed as a probe for RNA delivery check. (Labome)
FITC labeling of Biotin, Avidin, Lectins
FITC can be easily conjugated with antibodies against tags such as FLAG, His, c-Myc, and with several others.
In 2011, SJ Fleishman used FITC-conjugated c-Myc antibody in his flow cytometry computational experiment for the treatment of influenza. In similar research, FITC conjugated His-tag antibody was used to build transgenic fungi capable of killing mosquitoes in an immunocytochemistry test.
FITC is also able to perform highly targeted detection when coupled with avidin or streptavidin. Both Avidin/streptavidin and Biotin may be linked to FITC since they have amine variants available. Previously, an avidin-FITC conjugate was utilized to carry out immunofluorescence assays in order to investigate the role of mast cells in vascular leakage induced by the dengue virus. In immunohistochemistry, the same conjugate was also employed to discover characteristics of germinal cell lineages in Schistosomes (Labome). You can learn more about biotinylation in our related article.
Lectins are proteins that show high binding affinity towards carbohydrates. Many studies have demonstrated staining of plasma membranes using FITC-conjugated Triticum Vulgaris lectin (Emde et al.). B4 (a fluoresceinated isolectin) has widely been used for the staining of vascular endothelial cells.
Commercial FITC Protein Labeling Kits
| Product Name | Supplier | Approximate Price |
| FITC Conjugation Kit (Fast) – Lightning-Link® (ab188285) | abcam | ~$410 |
| FITC Conjugation Kit – Lightning – Link ® (ab102884) | abcam | ~$500 |
| Protein FITC Labeling Kit (ab288089) | abcam | ~$180 |
| EZLabel™ Protein FITC Labeling Kit | BioVision | ~$310 |
| FITC Labeling Kit | Sigma Aldrich | ~€380 |
| Fluoro Tag ™ FITC Conjugation Kit | Sigma Aldrich | ~€540 |
| Protein FITC Labeling Kit (BN01049) | Assay Genie | ~€510 |
| Antibody/Protein Labeling Kit – FITC | Biologicals | ~£480 |
| Rapid FITC Antibody Labeling Kit | ReadiLink™ | ~$240 |
