Histidine is an essential amino acid with interesting structural and chemical characteristics that allow protein conjugation. However, these modifications can be labile when trying to do in vivo approaches or require particular conditions. As a result of these difficulties, researchers find it challenging to achieve selective and direct covalent histidine bioconjugation.
Histidine bioconjugation techniques include the use of thiophosphorodichloridate reagents, bis-alkylation reagents, and C−H alkylation with C4-alkyl-1,4-dihydropyridine (DHP) reagents under visible-light-promoted conditions.
Histidine bioconjugation has remained less explored than other protein bioconjugation technologies targeted for some amino acids such as cysteine, tyrosine, and lysine. Nonetheless, in this article we’ll provide you methods for conjugation with Histidine so you have a starting point.
Histidine Bioconjugation Methods
Despite histidine bioconjugation being explored to a lesser extent, there are still various methods discussed in academic studies. Here we discuss three of them.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Method 1. Thiophosphorodichloridate Reagents For Chemoselective Histidine Bioconjugation
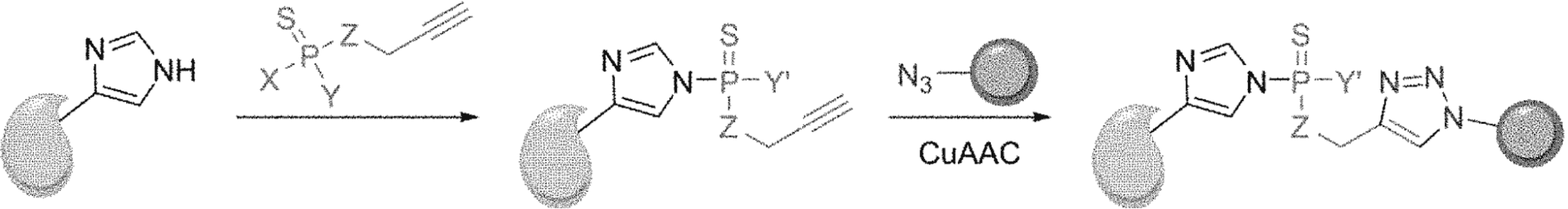
In this article, researchers utilized thiophosphoro alkyne dichloridate (TPAC), a thiophosphorodichloridate reagent, for chemoselective histidine bioconjugation as an alternative to enzymatic post-translational histidine phosphorylation. Once histidines are labeled, copper-assisted alkyne-azide cycloaddition (CuAAC) can be performed to add different payloads resulting in stable modifications of both single histidine residues or polyhistidine tags.
Here are the steps they followed.
Step 1. Design and Synthesis.
Researchers synthesized various phosphorus electrophiles until they found those that were able to resist hydrolysis. TPAC was chosen among others also because it produced the highest yield of bioconjugate when labeling histidine.
Step 2. TPAC Bioconjugation With Model Proteins.
Next, they labeled histidines on intact protein substrates such as ribonuclease A and small molecule amino acid models. Then they performed LC-MS/MS, collision-induced dissociation (CID), and Electron transfer dissociation (ETD) to analyze the modification site. Once they found that ribonuclease A had insignificant activity loss when treated with TPAC, labeling histidine residues were subsequently investigated in other proteins and HeLa lysates.
Step 3. Copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC)
After isolating those TPAC-modified reaction products with a strong cation exchange column, CuAAC was performed by adding two different payloads for detection and enrichment. We’ve discussed azide bioconjugation reactions and orthogonal bioconjugation techniques like click chemistry in other articles.
Step 4. TPAC Bioconjugation on His-tag for Protein Delivery.
Finally, the researchers utilized confocal microscopy to observe that TPAC provides covalent, histidine-selective bioconjugation that is functional inside living cells. They observed intracellular fluorescence of both functionalized His10tag-GFP (green fluorescent protein) and His6tag-mCherry protein.
Method 2. Site-Selective Protein Conjugation At Histidine
In this article, researchers used a bis-alkylation reagent (PEG(10kDa)-mono-sulfone) for histidine bioconjugation to insert different His-tags along an interferon-α2-a (IFN) without losing in vitro antiviral activity.
Here are the steps they followed.
Step 1. Preparation of Truncated N-terminal His-tag IFN analogs.
They prepared four variants of the typical six histidine residues (His6tags) used in expression studies at the N-terminal region of the IFN. Then they added glycine for molecule flexibility, so conjugation was more straightforward. They found that all variants had biological activity but decided to use only those with two histidines. (Tag1, 3, and 4 shown below).
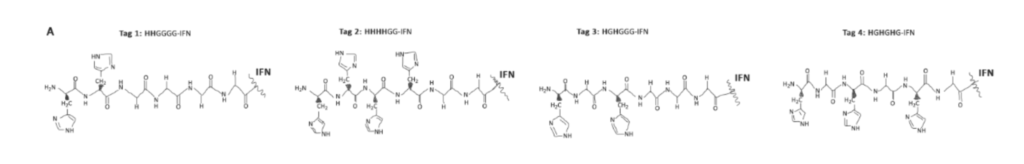
Step 2. Histidine Bioconjugation with Peg-Mono-Sulfones
Conjugation of the truncated His variants with PEG10-mono-sulfone instead of PEG10-bis-sulfone was preferred to avoid lysine conjugation under slightly acidic conditions, thus increasing the selectivity sought. After many studies, they found that the ideal conditions where mono-PEGylation could be performed in all three IFN histidine variants were at pH 5.0, protein concentration of 1 mg mL-1, and five eq. of PEG10– mono-sulfone 3 for an incubation period of 16 h at 20°C.
Step 3. Site Selection On IFN For Histidine Conjugation
The researchers also developed five different IFN variants with internal His-tags – replacing some amino acids in the original primary structure of IFN. Also, these locations were engineered considering how IFN binds with its dimeric receptor to maintain the biological activity.
After analyzing the in vitro antiviral activity, they decided to continue only with three IFN variants.
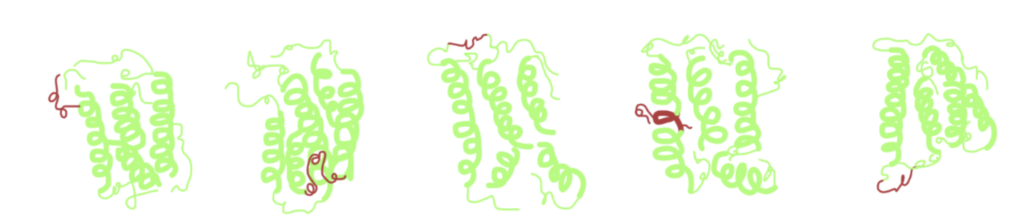
Step 4. Site-Selective Conjugation Studies
At this point, conjugation conditions on internal His-tags were replicated from the ones used for N-terminal His-tags. Mono-PEGylated IFN variants were produced in yields from 21-33%, considered an excellent recovery.
Moreover, all three PEG-IFN conjugates were biologically active, but the conjugation site does affect this activity.
Method 3. C−H Alkylation Of Histidine Using C4– Alkyl-1,4-Dihydropyridine (DHP) Reagents Under Visible-Light-Promoted Conditions
In this article, researchers utilized various DHP reagents to site-selectively modify Histidines via radical-mediated C2-selective C-H alkylation of the imidazole group by a Minisci-Type reaction. They started analyzing this method on simple Histidine derivatives until they established the conditions for more complex peptide substrates with varied lengths, compositions, and activities such as saralasin and His-containing peptide drugs such as angiotensin II, secretin, ubiquitin, bremelanotide, and Bleomycin A2. Moreover, in this study, chemo-selectivity was prioritized, strong oxidants were eliminated to avoid oxidation of peptide substrates, and the nitrogen groups from the imidazole ring of the modified histidines were conserved.
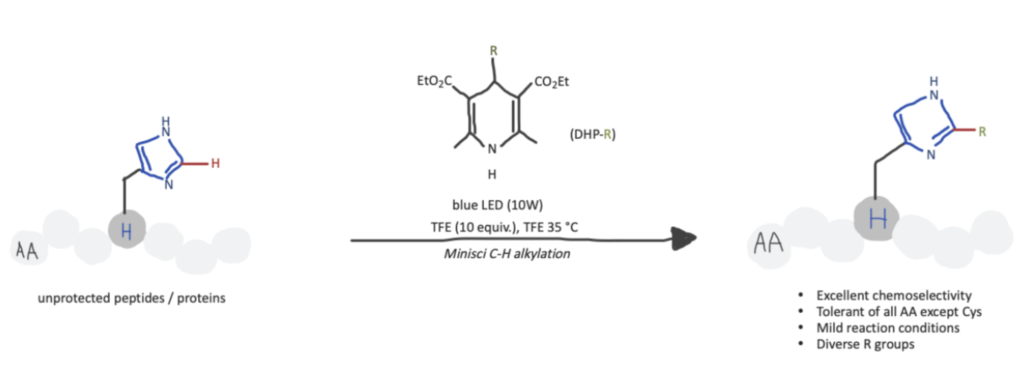
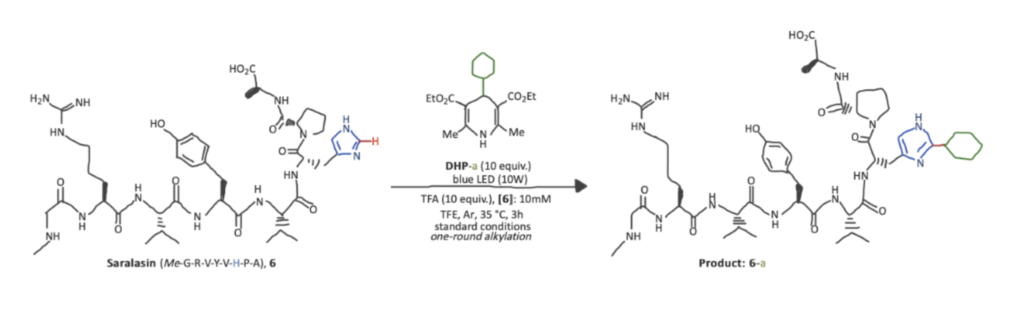
Step 1. Standard Conditions For Histidine Bioconjugation with His-Specific Alkylation
Based on experimental evidence from a previous work of Gary A. Molander and coworkers, standard conditions for histidine conjugation in Saralasin, an antihypertensive octapeptide, were established as follows: peptide (2.0 μmol, 1 equivalent), DHP (20.0 μmol, 10 equiv), Trifluoroacetic acid (TFA) (20.0 μmol, 10 equiv) in Trifluoroethanol TFE (0.2 mL, 10 mM) under irradiation of blue LED (10 W) under argon (Ar) atmosphere at 35 °C for three hours. After treating the reaction mixture with cold diethyl ether, the precipitate was collected and analyzed by liquid chromatography-mass spectrometry (LC-MS) to establish the yield of the desired product, a conjugated Salarasin.
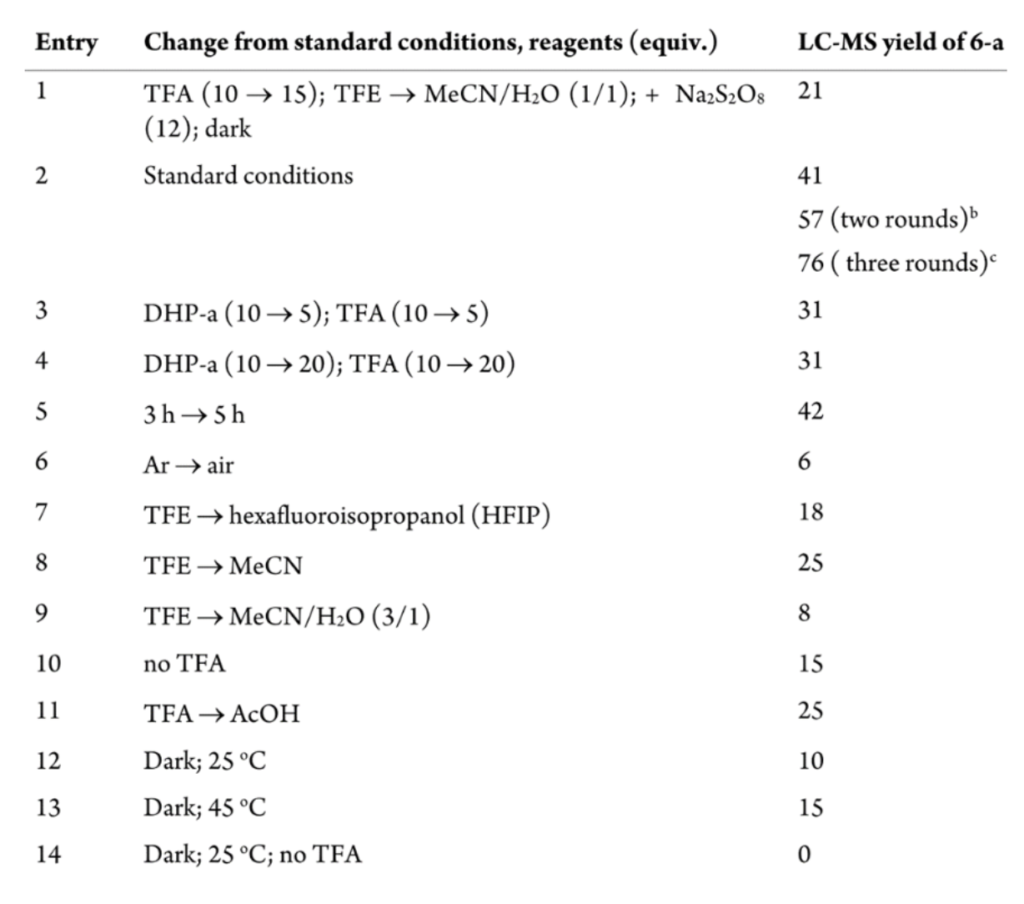
Step 3. Optimization of three-round Minisci-Type C−H Alkylation Protocol.
Researchers then questioned whether the conjugation of Saralasin could be improved by changing some conditions or quantity of reagent, as the first entry shows in the image below. However, they found that subjecting the precipitate of the first reaction mixture to a second alkylation procedure with the same conditions increased the yield significantly, and carrying out a third round of alkylation provided the highest yield, thus establishing the three-round alkylation protocol as optimal for this Minisci-Type reaction.
The remaining entries confirmed that TFE as a solvent, TFA, and LED irradiation were the best conditions and reagents for this protocol.
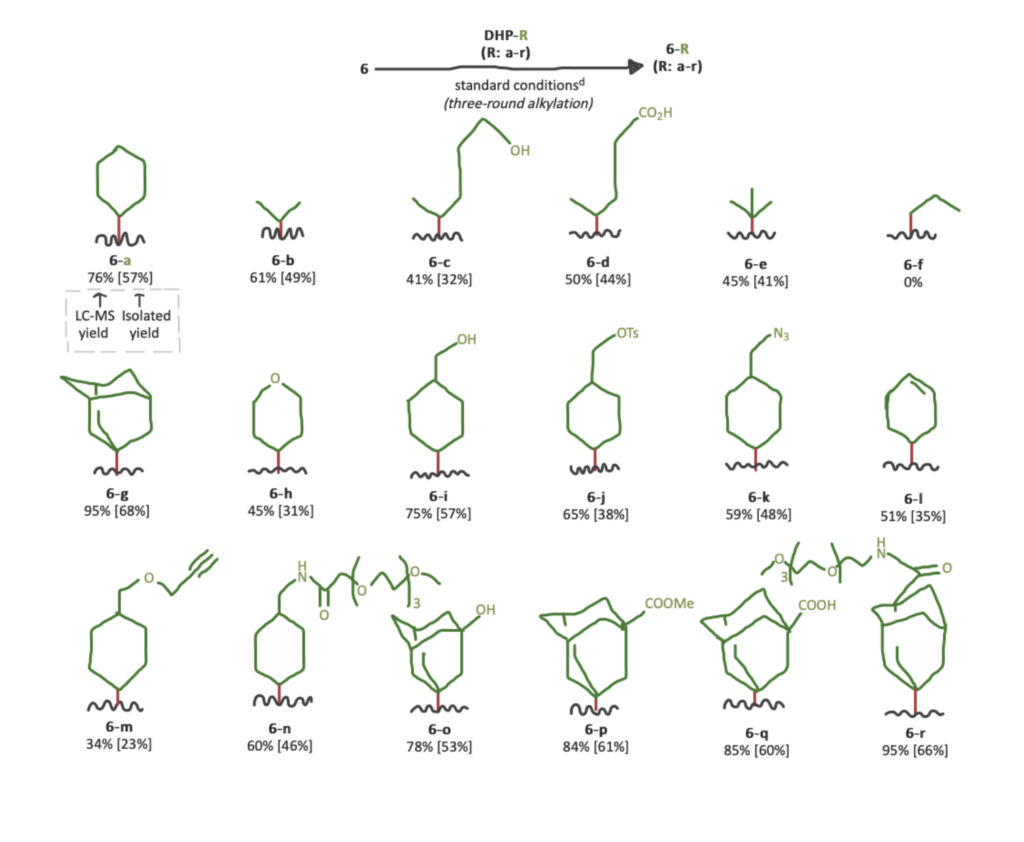
Step 4: Reactions of Saralasin with DHP reagents carrying different C4-alkyl groups.
Finally, they evaluated the optimized protocol with different DHP reagents. The main objectives were maintaining high chemoselectivity and forming fewer side-products. The chosen reagents can be easily prepared in a single step in high yield from a three-component reaction of corresponding alkylated aldehyde, ethyl acetoacetate, and ethyl 3-aminocrotonate.
Applications of Histidine Bioconjugation
Applications of histidine bioconjugates vary, but two of the most relevant are that they can be used in pharmaceutics and diagnostics independently of the approaches. For more broader applications of amino acid bioconjugation, arginine bioconjugation is able to interact with other amino acids and biomolecules.
Applications of histidine bioconjugation include modifications of polyhistidine tags, bioconjugation of therapeutic proteins, and labeling of histidine residues.
Application 1. Bioconjugates On Polyhistidine Tags For Recombinant Proteins
In the article described in Method 1, the researchers used a thiophosphorodichloridate reagent for chemoselective histidine bioconjugation so that they could study post-translational phosphorylation. This method can be used to react with polyhistidine tags as well as histidine residues in protein primary structures.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Application 2. Bioconjugation of therapeutic proteins.
In the article described in Method 2, the researchers PEGylated histidine residues both on N-terminal and internal His2tag of interferon-α2-a (IFN) to increase the activity, compared with simple PEGylation, to use it as an antiviral therapeutic protein. They utilized histidine because PEG bis- and mono-sulfones can conjugate with His-tags. Purification is also simple, so you can get high yields.
Application 3. Labeling of histidine residues
In the article described in method 3, the researchers utilized C-H alkylation for histidine bioconjugation to build diverse peptides for functional studies. Many peptide substrates can undergo this process, such as peptide drugs of 8−10 amino acids, complex bioactive peptides with 24−76 amino acids, and cyclic peptides like Bremelanotide.
They utilized histidine because these residues aren’t common in native proteins, thus providing an excellent site-selective target for peptide modification. Also, the heteroaromatic imidazole side chain has essential roles in protein function.