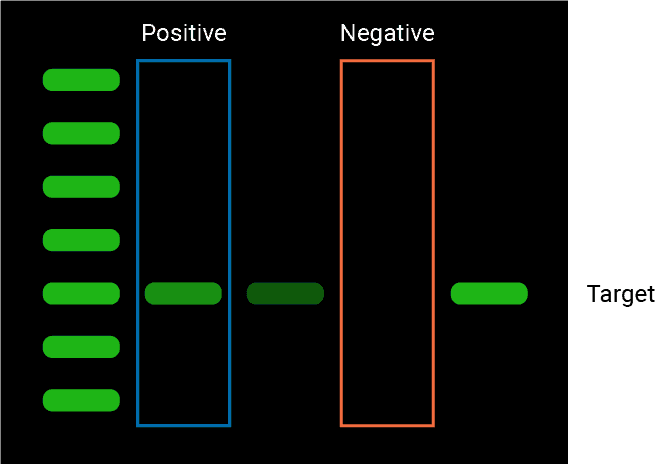
Western blot negative controls are essential in order to validate the results of the experiment. The presence of a negative control ensures that the bands observed in the western blot are indeed bands corresponding to the protein-of-interest, and not simply non-specific binding of the antibody to other proteins in the sample.
To choose the best western blot negative control, first assess the experimental conditions, then alter those conditions to remove the expression of your protein-of-interest while ensuring careful technique handling and appropriate antibody titers are used for immunodetection.
What Is A Western Blot Negative Control?
A western blot negative control for protein analyses is a sample taken from a cell line, tissue sample, or experimental condition that does not express a protein-of-interest.
A western blot negative control is a sample that is known to not contain the protein-of-interest, and is important for ruling out any false positive results. If any bands are observed in the negative control lane, this would indicate that non-specific antibody binding is occurring, since the sample does not contain any target protein (Mahmood and Yang, 2012).
For example, if your experimental sample is collected from a certain cell line or tissue culture, creating a mutant of that cell line or tissue culture that does not express your protein-of-interest would serve as good negative control. This negative control would keep all other variables constant while removing the presence of your target protein (Nie et al., 2017).
In the case of in vitro work, using co-immunoprecipitation, a protein-protein conjugation technique would require performing the assay simultaneously under two conditions: one in the presence of your target protein, and the other in its absence. Samples would be collected from both conditions, and the sample lacking the protein-of-interest would be considered the negative control.
How To Pick A Negative Control
Step 1. Assess The Experimental Conditions
The first step to decide on your negative control is to assess your experimental conditions. What is the background in which the experiment is being conducted? Is the sample being collected from a specific cell line or tissue culture? Or is it an in vitro experiment with artificial conditions? Once the background conditions are established, Step 2 analyzes how to alter them to generate a negative control.
Step 2. Alter The Experimental Conditions To Remove The Target Protein
Once the experimental conditions are established, a negative control can be created by altering a single variable: the presence of your target protein. Importantly, all other variables should remain constant in order to maintain the accuracy of your negative control. The closer the conditions of your negative control are to the experimental conditions, the greater the validity of your experiment. As discussed previously, depending on your experimental conditions, a negative control can be generated either by creating a mutant cell line or tissue culture, or by performing the in vitro assay in the absence of your target protein.
What Is A Western Blot Positive Control?
A western blot positive control for protein analyses is a sample taken from a cell line, tissue sample, or experimental condition that is known to express a protein-of-interest.
A positive control in a western blot ensures that your target protein is being properly detected. The positive control is a sample that is known to contain the protein-of-interest, and therefore expected to contain a protein band. If a band is not observed in the positive control lane, then that would be an indicator of faulty conditions that compromise the results of the entire experiment. For more information, refer to this article.
For example, if your experimental sample is collected from a certain cell line or tissue culture, a positive control could be generated by creating a mutant version of the cell line or tissue culture that constitutively expresses the target protein. Alternatively, fusing the target protein to another known protein, through protoamine bioconjugation methods or enzyme-catalyzed bioconjugation, could also produce a positive control. In the case of in vitro work such as co-immunoprecipitation, a positive control could simply be a sample of purified protein-of-interest that is collected separately.
Why You Should Use A Western Blot Loading Control
Use a western blot housekeeping protein to ensure adequate sample loading, normalize your samples for qualitative and quantitative analysis, and correct for the edge effect.
Loading controls in a western blot allow for the normalization and subsequent comparison of signals between samples. Without normalization, the intensity of protein bands in different samples cannot be compared, but a loading control permits that kind of analysis (Mohan et al., n.d.).
Reason 1. Validates Sample Loading
Because housekeeping proteins are abundant in most cell types and are constitutively expressed, checking the intensity of their bands on the western blot ensures that equal amounts of the samples were loaded into each lane. If there is a significant variation between the amount of housekeeping protein between one sample and another, it could be indicative that the samples were not loaded equally onto the gel, or not transferred equally onto the blot, and your experiment may need to be repeated.
Reason 2. Normalizes Samples
There will naturally be some minor variation between the protein content of different samples, simply due to human and measurement error during the experimental procedure. These variations can be accounted for by quantifying the loading control bands and using them to normalize the data between samples. Since housekeeping proteins are so abundant and constitutively expressed, they can be used as a baseline to analyze any differences between the band intensities of your target protein. For more information on western blot normalization, refer to this article.
Reason 3. Accounts For Edge Effect
One final use for loading controls in western blots is to account for the edge effect, which is when outer lanes on the edges of the blot have more intense bands than those in the middle. Since loading controls are expected to have equal expression, a pattern of variation in loading control bands where those on the outer edges are more intense and those in the middle are fainter can be attributed to the edge effect. This can then be accounted for when analyzing the bands of your target protein, to ensure that any variations in intensity are not solely due to the edge effect.
Choosing The Best Western Blot Housekeeping Protein
Although commonly used, sometimes β-actin is not a reliable loading control in western blot analysis because its expression level can be affected by certain pathologies, and high β-actin levels can inhibit quantitative analyses.
Housekeeping genes are commonly used as loading controls because they are genes that are ubiquitously expressed in many different cell types and conditions in abundant amounts. When it comes to selecting an appropriate housekeeping protein to use as a loading control, some factors to consider include the protein’s stability of expression, molecular weight, and cellular localization. Generally, you want a housekeeping protein that is stably expressed under all conditions in which your samples are being collected. Additionally, the housekeeping protein should be of a different molecular weight than your target protein, to ensure that the bands of each are distinct. Finally, it is advisable to select a housekeeping protein with the same cellular localization as your protein-of-interest so that during sample collection and preparation, both are present in abundant amounts. Some examples of common housekeeping proteins used as loading controls include: β-actin, HDAC1, GAPDH, and β-tubulin (Dittmer and Dittmer, 2006; Nie et al., 2017).
Common Western Blot Housekeeping Proteins
| Housekeeping Protein | Cellular localization | Molecular Weight (kDa) |
| β-actin | Cytoplasmic | 43 |
| HDAC1 | Nuclear | 62 |
| GAPDH | Cytoplasmic and Nuclear | 36 |
| β-tubulin | Cytoplasmic | 50 |
Troubleshooting Western Blot Control Problems
Common western blot control problems include the target protein band showing up in the negative control, the target band failing to be in a positive control, and overlapping bands between the target protein and housekeeping controls.
Problem 1. Negative Control Shows Target Protein Band
If your negative control shows a band where your target protein is expected to be, this could be for a few reasons. First, there may have been contamination of the negative control by protein from the experimental sample. This can be remedied by more careful handling of samples to avoid any cross-contamination. Alternatively, the signal in the negative control may be due to the non-specific binding of the antibody to other proteins in the sample. If this is the case, using lower antibody titers, or a different antibody conjugation method, and more stringent membrane blocking may solve this issue.
Problem 2. Positive Control Does Not Show Target Protein Band
If your positive control does not show a band for your target protein, this could be indicative of an issue in your western blot technique. There are many stages where such an error could occur. For example, there may have been an inadequate transfer of protein from the gel to the membrane, due to the transfer time being too short or too long. Alternatively, your washes may have been too stringent and removed the protein from the membrane, making it undetectable. Finally, it could be an issue with the antibodies, which may not have been sensitive enough, or were used at a suboptimal concentration.
Problem 3. Protein-Of-Interest And Housekeeping Control Bands Overlap
If the molecular weights of the protein-of-interest and the housekeeping proteins are too close, then a different housekeeping protein should be selected for a clearer band analysis to prevent overlapping.
