Bioconjugation is an important technique for creating new molecular entities with therapeutic applications. Biomolecules including peptides, proteins, and antibodies are attractive carriers for selective targeted treatments because of their biocompatibility and specificity. Therefore, new molecular units must be developed to meet the demands of next-generation targeted therapies. Bifunctional linkers are amenable solutions as they are readily able to undergo chemical modifications to facilitate a variety of bioconjugation reactions. In particular, bioconjugation linkers based on a N-alkylated α,α-dialkyl dipeptide are fairly stable and have immense use in designing antibody-drug conjugates. Ultimately, careful considerations must be taken when choosing bioconjugation linkers.
Bioconjugation linkers are important molecules that combine different groups together and have important implications for antibody-drug carrier designs. Common bioconjugation linkers include NHS and carbodiimides, which facilitate amine-to-amine and amine-to-carboxyl crosslinking, respectively.
Types Of Chemical Linkers
Chemical linkers are molecules that connect two or more groups. Common categories of linkers include those that are cleavable or non-cleavable, bifunctional, homo- or hetero-bifunctional, trifunctional, and photocleavable.
Cleavable And Non-Cleavable Linkers
Cleavable and non-cleavable linkers have properties that are favorable for intracellular drug release and, so, are commonly used to develop antibody-drug carriers. How these linkers are chosen is contingent on the drug and its target, as well as the intracellular processes to which the bioconjugate is exposed to. Examples of cleavable linkers include those derived from hydrazones, disulfides, or enzymatically-cleavable peptide scaffolds (Ramos-Tomillero et al., 2020). A commonly used non-cleavable linker for bioconjugation reactions is succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate.
Bifunctional Linkers
Bifunctional linkers cause covalent connections to form between proteins that are close in proximity. A bifunctional molecule with two reactive ends joined by a spacer, commonly containing a disulfide bond, might be used as a cross-linker. The disulfide bridge is split, the cross-linked pairs are freed, and they can be detected when a reducing agent is applied. Choosing the right bifunctional crosslinker is important, and the most common ones are those with photoreactive groups that turn into reactive fluorophores when exposed to UV light, resulting in photolabeling of the cross-linked moieties (Phizicky and Fields, 1995; Fancy, 2000).
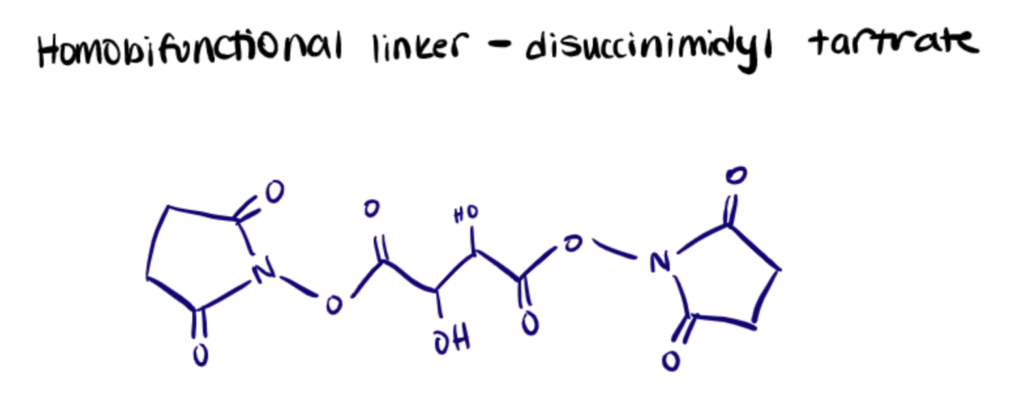
Homobifunctional Linkers
Homobifunctional linkers are composed of two identical reactive functional groups separated by different lengths. The reactive endpoints are chosen for their chemoselective characteristics, which frequently target proteins’ main amines and sulfhydryl groups. Dimethyl pimelimidate dihydrochloride, suberic acid bis(N-hydroxysuccinimide ester), and disuccinimidyl tartrate are popular examples.
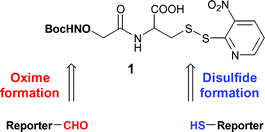
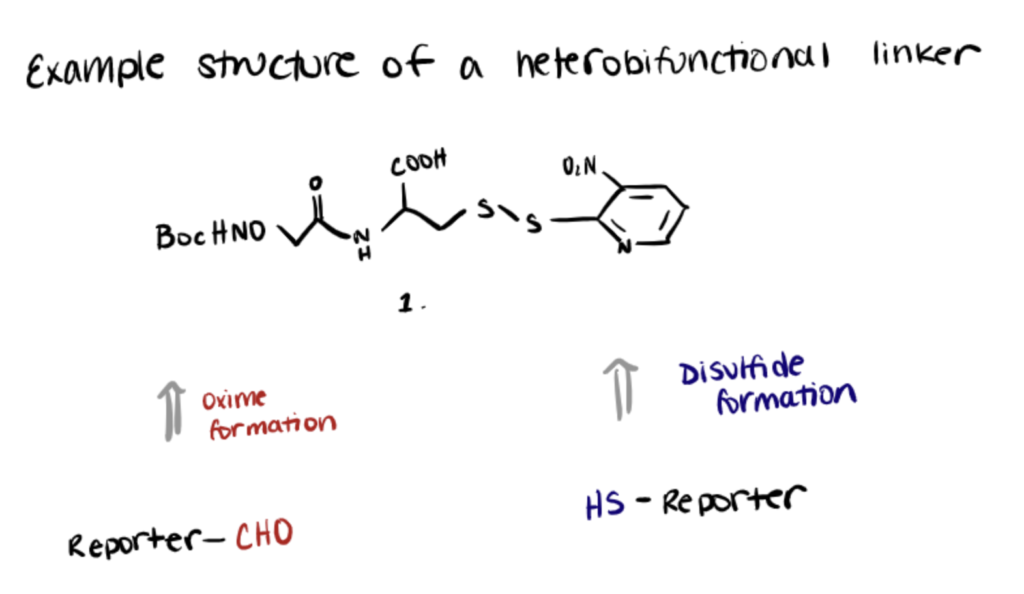
Heterobifunctional Linkers
Heterobifunctional linkers possess two distinct reactive groups that can be utilized to connect diverse functional groups. Using dissimilar biomolecules, these chemicals are used to create numerous intermolecular bioconjugates. Compared to homobifunctional crosslinkers that only allow for one-step molecule conjugation, heterobifunctional crosslinkers allow for two-step molecule conjugation, which reduces the risk of unwanted polymerization or self-conjugation.
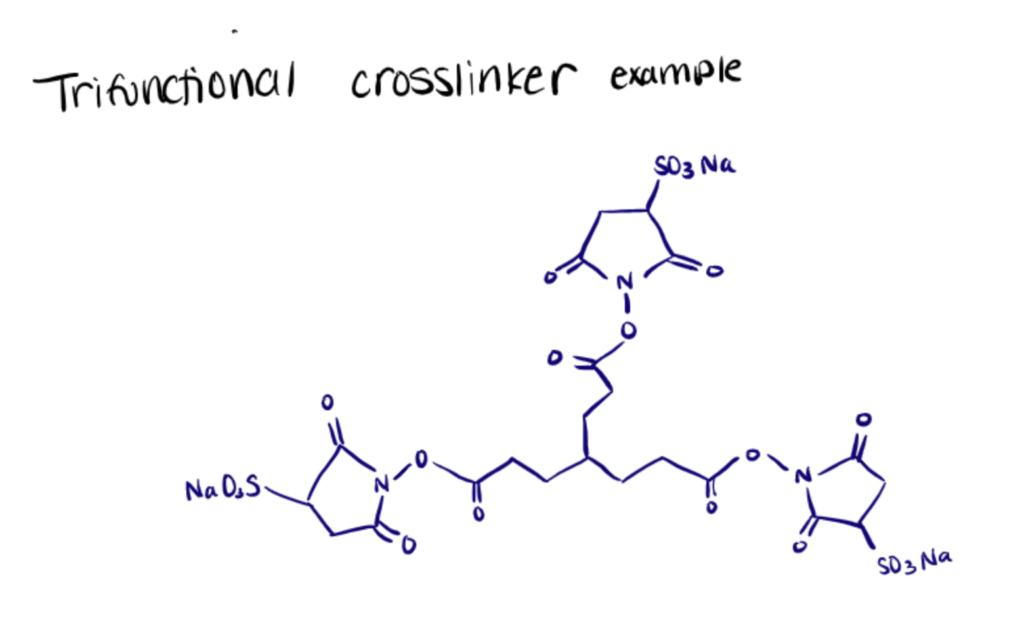
Trifunctional Linker
Trifunctional linkers are useful for identifying protein interactions. For the initial targeting of a known ‘bait’ protein, one arm of the trifunctional linker typically consists of a cleavable spacer and a chemoselective reactive group used for protein bioconjugation.
Photocleavable Linkers
Photocleavable linkers can be cleaved on demand by using light, and are frequently utilized as DNA or RNA-based catalysts for the construction of multi-functional single-stranded nucleotide conjugates.
Types Of Bioconjugation Linkers
Bioconjugation crosslinking techniques include amine-to-amine linkers, such as N-hydroxysuccinimde, and amine-to-carboxyl linkers like carbodiimides.
N-Hydroxysuccinimide: An Amine-To-Amine Linker
N-hydroxysuccinimide (NHS) bioconjugation involves the linking of an amine-reactive group with the primary amines of a protein or a biomolecule. Various fluorescent probes, biotin, and cross-linkers can be conjugated to primary amines via NHS chemistry (Nanda and Lorsch, 2014). Below is an example workflow on using NHS ester as an amine-to-amine linker.
Step 1. Determine The Required Amount Of Bifunctional Linker With NHS Esters
NHS ester linkers have the potential for mono-labeling and are suitable for a wide range of proteins and peptides. In some circumstances, however, performing the reaction with more or less NHS ester depends on conjugate protein solubility, structure and reagent.
Step 2. Dissolve NHS Ester In Amine-Free Solvent
NHS ester should be dissolved in 1/10 reaction volume of DMF or DMSO. DMF without amines is the chosen solvent. NHS ester can be kept in solution for 1-2 months at -20°C after the reaction.
Step 4. Prepare Biomolecule Solution
Biomolecules should be dissolved in a 9/10 reaction volume of buffer with a pH of 8.3-8.5.
It is vital to remember that pH is the most critical factor. Avoid using amine-containing buffers. Note that when undertaking large-scale labeling, the mixture tends to acidify with time due to NHS ester hydrolysis. The pH should be monitored, or a more concentrated buffer should be used.
Step 5. Combine The NHS Ester With Dissolved Biomolecules
Vortex the NHS ester solution into the biomolecule solution for N-terminal bioconjugation. Keep on ice overnight or for at least 4 hours at room temperature.
Step 6. Purify The Crosslinked Bioconjugate
For macromolecules, gel-filtration is the most general. Another option is precipitation and chromatography. Organic impurities (such as N-hydroxy succinimide, NHS ester, and hydrolysis-produced acid) are usually always easy to separate. Proteins and nucleic acids can be precipitated with ethanol or acetone (Lumiprobe).
Carbodiimide: An Amine-To-Carboxyl Linker
Carbodiimides are commonly used for EDC bioconjugation to crosslink carboxyl groups with an amine. The most popular carbodiimide is EDAC because of its water-solubility, which is highly useful in various applications. Below is an example workflow on using EDAC as an amine-to-carboxyl linker.
Step 1. Prepare The Carrier Protein
Desalting may be required to remove unsuitable buffers or interfering substances like thiols, amines, and acetates. CelluSep dialysis or desalting columns can be leveraged.
Step 2. Prepare The EDAC
A working solution of 100 mM should be prepared and used immediately.
Step 3. React The EDAC With The Carrier Protein Mixture
The EDAC will activate the carboxyl groups in the carrier protein and an amine-containing molecule can be added (in excess) to then react with the carrier mixture. This reaction may need to be optimized depending on their respective chemical and molecular properties. Incubate for 2 to 3 hours at room temperature. The conjugate may be frozen until use.
