Primary amines (–NH2) are the most common functional groups present in virtually all proteins, peptides, as well as a host of other macromolecules. They exist at the N-terminus of each polypeptide chain (α-amino group) and in the side-chain of lysine amino acid residues (ε-amino group). These amino groups are especially nucleophilic and occur predominantly on the solvent-exposed outside surfaces of native protein tertiary structures — making them easy targets for the conjugation. In this article we will discuss NHS ester bioconjugation techniques which target primary amines.
NHS Ester bioconjugation with amine-containing biomolecules leads to the creation of a stable amide bond between the two target molecules. To react an NHS ester with an amine, mix the amine and NHS-ester in a polar aprotic solvent like DMSO at a pH where the amine is deprotonated.
First introduced by Anderson et al. in 1963, N-hydroxysuccinimide activated esters (NHS esters) are today considered as one of the most powerful amine-specific functional groups that are incorporated into commercially available bioconjugation reagents.
Not only are they relatively easy to prepare, but NHS esters have also been shown to form conjugates with excellent stability and biocompatibility.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
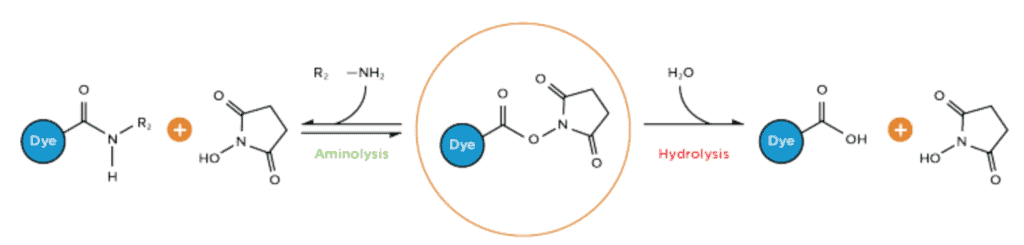
NHS Ester Bioconjugation Reaction with Amine
NHS-esters react readily with compounds containing amino groups, forming a chemically stable amide bond between the labeling reagent and the target molecule. The reaction is strongly pH-dependent, mainly due to the varying degrees of the protonation of the amine at each pH.
NHS Ester bioconjugation reactions with amines are pH dependent. Deprotonated primary amines are the most nucleophilic among the available functional groups present in a typical protein, and their reactivity decreases as the level of protonation increases.
At lower pH values, the peptide’s N-terminal amine is in its more protonated form, thereby rendering the functional group unreactive as nucleophiles— slowing the reaction rate and leading to less efficient conjugation. In these cases you might consider C-terminal bioconjugation instead.
Below we discuss a general method for NHS ester bioconjugation reactions with amines when used to tether antibodies to the surface. For a more detailed discussion read this article. You may also be able to react with amines using maleimides. We’ve covered this side reaction in our article on bioconjugation of maleimide.
NHS and Sulfo-NHS Esters are also used as stabilizing agents for EDC coupling reactions involving a crosslinker and a carboxylic acid. You can read more in our article, EDC bioconjugation. For other reactions involving amines, check out our article on bioconjugation using epoxides.
Step 1. Prepare Active Ester Surface
To construct an active NHS ester-containing surface for the selective bioconjugation with the reactant, an active monolayer surface was constructed by immersing a gold substrate in a 0.10 mM solution of 3,3′-dithiobis (succinimidyl) propionate (DSP) in ethanol. This created a monolayer of the gold-bound thiolate of DSP, 3-N-hydroxysuccinimidyl propanethiolate.
We’ve covered other methods for bioconjugation of nanoparticles made of gold or iron in a related article.
Step 2. React NHS-activated Substrate with Amines on Antibodies
The NHS-activated substrate was reacted with amines on antibodies in a buffered solution that maintained the deprotonated state of the antibodies. This led to immobilization of proteins on the surface via formation of stable amide bonds.
NHS esters are activated through spontaneous hydrolysis in the presence of water and/or moisture, the main bioconjugation buffer. In the reaction, the carbonyl group of the activated NHS esters is attacked by a nucleophilic primary amine. The coupling reaction releases NHS (a weak acid) and forms a tetrahedral intermediate with an amide bond (a very stable covalent linkage).
To yield stable amide bonds for bioconjugation, the buffers used to dissolve the target molecules must be conditioned to the optimal pH for amine reactivity (pH 7.2 to 9). Above this range, the reagent containing NHS esters will hydrolyse too rapidly in the presence of water (further discussed in section ‘NHS Ester Hydrolysis’). You can learn more about challenges with bioconjugation in water in our related article.
Step 3. Purify Immobilized Antibodies
While it will not react further, the hydrolyzed NHS ester may contribute to background noise during the quantification process. So, free unconjugated NHS esters, uncoupled substrate, and the NHS acid produced by hydrolysis were removed by washing.
If you are using free, non-immobilized conjugates, you can remove unconjugated NHS esters via HPLC, gel-filtration, precipitation, or chromatography (Koniev and Wagner, 2015). We have also discussed other, more specific antibody conjugation methods in our linked article.
NHS Ester Bioconjugation Reaction with Alcohol
NHS esters can react with other nucleophiles besides primary amines such as amino acids possessing primary alcohol side chains (-OH) if they are present and accessible on the target molecule.
Leavell et al. (2004) has successfully shown the formation of intramolecular N-terminus/lysine-tyrosine bond and tyrosine-tyrosine by using ethylene glycol bis[succinimidylsuccinate] (EGS) on an oxidized insulin β-chain under slightly acidic conditions (pH 6.0).
As reported by Mädler and Zenobi (2009) and Miller et al. (1997), serine and threonine require the presence of neighboring histidine residue in specific sequences to increase the reactivity of hydroxyl groups toward NHS esters.
This is mainly due to the increased nucleophilicity of the hydroxyl-containing side chains significantly, as previously observed for biotinylation experiments with NHS esters on Ser, Tyr and Thr (Miller et al., 1991; 1993; 1997). However, these side reactions possess largely decreased rates and the resulting ester bond has a somewhat lower stability than the amide bonds resulting from the acylation of amino groups.
Below we discuss a general method for NHS ester bioconjugation reactions with serine using a synthetic cross-linker 3,3-Dithiobis(Sulfosuccinimidyl Propionate), DTSSP. For a more detailed discussion read this article (link to article). You can also learn more about biotinylation techniques in our related article.
Step 1. Prepare Peptides to React with DTSSP
To understand the chemistry and crosslinking of serine side chain and NHS ester reagents, the authors prepared over 17 model peptides of BSA, CA, αB, and αA. For more information on how to choose the right bifunctional crosslinkers for conjugation reactions like DTSSP, read our other article.
Step 2. Mix Peptides with NHS-Ester Containing DTSSP in PBS
Peptide aliquots were diluted to 27 M in the phosphate buffer (100 mM, pH 6.7) and added with the DTSSP suspended in 30 mM DTT to initiate the reaction.
Step 3. NHS Ester Bioconjugation with Amines and Hydroxyls on Peptides
Intramolecular hydrogen bonds can form between the hydrogen of the side-chain hydroxyl group and the imidazole moieties from histidine. This reaction increases the nucleophilicity of the oxygen of the side-chain hydroxyl group from serine, threonine, or tyrosine. Mädler and Zenobi (2009) have also reported similar activity from the guanidinium group of arginine.
Even though Tyrosine and Serine amino acids don’t contain primary amine side chains, the authors demonstrated that 85% of the tyrosine residues and 40% of the serine residues reacted with DTSSP under the experimental conditions.
The histidine will rapidly react with the NHS ester, but since the intermediate acyl imidazole is highly unstable and also reactive toward nucleophiles, the DTSSP ends up reacting with the nearby –OH group to form an ester linkage.
This reaction does not take place as readily in aqueous solution without a histidine group present, because of competition with water, which is in much higher concentration and can promote hydrolysis of the NHS ester rather than ester formation with serine, threonine, or tyrosine. If you are trying to conjugate to less abundant amino acids like tryptophan bioconjugation, methionine-selective bioconjugation, or tyrosine-selective conjugation using electrochemical conjugation techniques, read our other articles.
NHS Ester Reactions in DMSO, an Organic Solvent
Bioconjugation reactions generally employ aqueous buffers to ensure stability of biomolecules like proteins. However, water can act as both an electrophilic and nucleophilic solvent, thereby reducing both the nucleophilicity and the base strength of nucleophilic amine groups via hydrogen bonding.
To increase the rate and yield of NHS ester reactions utilize DMSO, a polar, aprotic solvent which destabilizes the -OH group for better nucleophilic reactivity.
It’s possible to increase the rate of reaction by conducting NHS ester bioconjugation in organic solvents, mainly due to the desolvation of the amine nucleophile and/or stabilization of the transition rate (Vinson, 1967). Additionally, with the use of organic solvent, the spontaneous decomposition of NHS esters through hydrolysis could also be avoided (Koniev and Wagner, 2015).
Dimethyl sulfoxide (DMSO) is an important polar aprotic solvent commonly used in NHS ester bioconjugation to form clusters between NHS esters and the target molecule — provided the bioconjugation target is soluble and stable in such environments. Several studies on ester hydrolysis have concluded that the rate enhancing effect of DMSO is mainly through the destabilization of the -OH group for better nucleophile reactivity (Li, 2020; Kendall et al., 2015).
The mechanism of ester aminolysis in aprotic media was subjected to detailed analysis on several reports, starting with the classical work of Menger and Smith (1972). DMSO has been shown to react with -OH radicals in the liquid phase through three distinct oxidation pathways.
However, the main pathway involves the role of DMSO as a proton acceptor. The partial negative charge on the oxygen atom of the DMSO molecule favors the formation of hydrogen bonds with the primary amine group, which displays enhanced reactivity as a proton donor (Quienne et al., 2021; Jen et al., 2019).
Below we discuss a general method for NHS ester reactions in DMSO as a useful strategy to reduce the reaction time for post-synthetic modification of RNAs. For a more detailed discussion read this article.
A schematic representation showing coupling of amine-containing RNA with NHS-ester linked Tide Fluor 3 fluorophore. Image source Bioengineered.
Step 1. Synthesize Aminoallyl-labeled RNA
In the study, the author used aminoallyl-modified 71nt adenine riboswitches aptamer domain (rbA71) synthesized via PLOR as a model RNA to optimize the NHS ester coupling reaction for fluorescent labeling of RNAs.
Step 2. NHS-Ester Bioconjugation Between Fluorophore and RNA
Calculate required amounts of NHS-ester and dissolve them in amine-free DMSO. The authors used an NHS ester-linked Tide Fluor 3 (NHS-TF3) reagent to generate fluorescent-labeled rbA71.
The authors demonstrated that the optimal solvent combination to achieve efficient labeling of RNA is 45–55%, DMSO and NaHCO3 buffer (pH 7.0–7.5, 300 mM).
Due to the use of DMSO, the bioconjugation efficiency increased 2 times by using optimized conditions — shortening the reaction time to 0.5 h — which is more favorable to prevent RNAs from degradation.
The reaction profile has a greater stabilizing effect on the intermediates, transition states and product than on the reactant complex. When using DMSO as a solvent, the pH of the solution should be adjusted to 6.
NHS Ester Hydrolysis
It should be noted that the hydrolysis of NHS esters with the amine group is a competing reaction. The activated ester group of the NHS ester will hydrolyze spontaneously in the presence of water/moisture.
Hydrolysis of NHS ester is generally not an issue, as primary amines exhibit much higher nucleophilic reactivity compared to water. However, hydrolysis and/or degradation of NHS esters can occur before the attack of the amine — causing them to become non-reactive, reducing the number of reactive sites for aminolysis coupling, and decreasing the efficiency of the linking chemistry (especially under suboptimal reaction conditions) (Lim et al., 2015).
NHS ester hydrolysis can take place before an amine is able to attack, causing the NHS ester to become unreactive.
Thus, the coupling conditions must strike a balance between the rates of both the aminolysis and hydrolysis reactions. The rate of hydrolysis may be monitored by measuring the increase in absorptivity at 260 nm as the NHS leaving group is cleaved to yield ‘free’ dye (Hermanson, 2008).
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.