With recent advancements in nanotechnology the biomedical field has been able to improve many aspects of medicine using nanoparticles. Particles such as gold nanoparticles, iron nanoparticles, and quantum dots have been applied in many different areas of medicine to include bioimaging, fluorescent labeling, and biosensing applications. For these applications, the functionalization and bioconjugation of nanoparticles with proteins, nucleic acids, carbohydrates, and various other biomolecules is of utmost importance.
Bioconjugation of nanoparticles can be accomplished by using surface amines or thiols via chemisorption methods or by using physisorption methods such as hydrophobic interactions and Vander Waals interactions.
Common methods for bioconjugation of nanoparticles can be categorized into physisorption and chemisorption techniques. Physisorption bioconjugation is establish via electrostatic, hydrogen bonding, hydrophobic and Vander Waals interactions, while chemisorption relies on chemical modification of the biomolecule and/or nanoparticle followed by the formation of a new bond between the conjugates. While physisorption is simpler to accomplish, chemisorption allows for better control over conjugation preventing undesirable bond formation, spatial orientations, and conformation. For example, the sensitivity of a biosensor that utilizes antibodies to capture an antigen, on the surface of the nanoparticle is highly dependent on the spatial orientation of the antibody as it bonds to the nanoparticle.
Related articles:
- Nanoparticles can deliver radiopharmaceuticals for anti-cancer therapy. Read more about bioconjugation methods for radiopharmaceutical chemistry in our article.
- A discussion of magnetic nanoparticles and their applications can be found in our article, magnetic nanoparticle bioconjugation.
What are Nanoparticles?
In the realm of nanomaterials one of the most prominent is nanoparticles. Currently there is no standard definition for the size range of nanoparticles, some studies will define nanoparticles in the range of 1 to 1000 nm while other studies have their own definitions.
Nanoparticles are particles whose length in all three dimensions is less than 100 nm. These particles can be spherical in nature (nanospheres), spherical with points (nanostars), rod shaped (nanorods), and many other morphologies, each having their own advantages and applications.
Nanoparticles have other applications outside of the world of medicine as well. Applications such as UV blocking, composite reinforcement, and colloid stabilization. Their small size gives them many unique properties that cannot be achieved with larger particles, helping to improve material performance.
For example, quantum dots exhibit unique fluorescent properties due to a phenomenon called quantum confinement which is typically only seen in nanoparticles smaller than 10 nm. As the particle diameter decreases the number of conduction electrons decreases, reducing the conduction band and increasing the band gap. By fine tuning particle sizes, it is possible to control the size of the band gap and what color the quantum dots will fluoresce at. You can learn more about bioconjugation of quantum dots in our related article.
Different Types of Nanoparticles
Along with different morphologies, there are also many types of nanoparticles made from several materials.
Different types of nanoparticles include gold nanoparticles, magnetic iron nanoparticles, silver nanoparticles, and many others.
Gold Nanoparticles
Gold nanoparticles are conventionally made via the reduction of chloroauric acid which can be mediated with chemicals like borohydride and CTAB to influence the size, morphology, and characteristics of the particles. New research has begun to investigate polymer mediated synthesis routes, UV-induced photochemical synthesis, and many others to better control the size, morphology, and colloidal stability of particles.
These nanoparticles have many applications to include biosensing, targeted cancer treatments, and bioimaging. One of the most exciting advancements is in bio-imaging. Because of the nanoparticles’ small size and the characteristics of gold, these particles can produce strong electromagnetic radiation via surface plasmon resonance. This phenomenon allows the particles to be used for the enhancement of NIR, FTIR and SPR systems. By labeling biomolecules such as antibodies it is possible to improve the sensitivity of SPR systems to allow for more sensitive detection systems. This has many applications in the medical field for things like HIV and COVID testing.
If you want to learn more about gold nanoparticles, our article, nanobody bioconjugation highlights an application of gold nanoparticles conjugated to nanobodies.
Iron Nanoparticles
Iron nanoparticles can be formed via the decomposition of iron pentacarbonyl, sonochemical decomposition of iron carbonyl, and many other methods. These nanoparticles have very interesting properties, for example when iron nanoparticles are formed in the 10 – 20 nm range they have a property called super-magnetism. This makes the iron nanoparticles ideal for magnetic recording media used in electronics and many other applications. Additionally, iron nanoparticles can act as oxidizers making them great catalysts for the catalytic upgrading of fuels, such as the conversion of coal or natural gas to syn-gas.
Bioconjugation of Gold Nanoparticles
One of the most common uses of gold nanoparticles is for bio-imaging. This typically requires the bioconjugation of the nanoparticles to an antibody. For example, gold nanoparticles/antibody conjugates can bind to an antigen and enhance fluorescence improving HIV detection.
Common Functional Groups on Gold Nanoparticles
The methods used to synthesize gold nanoparticles can affect the type of functional groups present on the surface and the surface chemistry involved in bioconjugation is highly dependent on these groups.
Common functional groups on gold nanoparticles include amines and thiols. By taking advantage of the high affinity these groups have for gold surfaces, ligands can be attached to the gold surface, allowing for conjugation of the nanoparticles with biological molecules such as oligonucleotides, proteins, and antibodies.
Amine-Mediated Bioconjugation of Gold Nanoparticles
Amine functionalized gold nanoparticles are soluble in water and have a positive charge on their surface. One application of amine functionalized gold nanoparticles is in drug delivery. The positively charged amine groups can interact with negatively charged nucleic acids helping to prevent undesired interactions, allowing targeted cell delivery. One study used amine functionalized nanoparticles along with PEGylated siRNA for stabilized delivery of siRNA. We’ve discussed pegylation of gold nanoparticles more in our article.
Polymers can be conjugated to proteins and antibodies using a range of functional groups such as amines, carboxyls, or thiols. Explore conjugation kits for polymers, proteins, and antibodies, here.
Thiol-Mediated Bioconjugation of Gold Nanoparticles
Because of gold’s high affinity to thiol groups and the ability of thiol containing ligands to assemble on the gold surface in a dense special arrangement, thiol functionalized gold nanoparticles are a simple and efficient method of surface functionalization. There are many sulfur-containing ligands allowing for a diverse number of conjugations to happen. One study took advantage of thiol containing ligands to conjugate the surface with the glucose oxidase enzyme for the biosensing of glucose levels. Side Note: For reactions to thiols on proteins, you can use palladium-catalyzed bioconjugation. We’ve also covered cysteine bioconjugation techniques in detail in another article (cysteines have thiol groups).
Method for Conjugation to Amine Groups on Gold Nanoparticles
Amine functionalized gold nanoparticles can be linked using many different reactions. One such reaction takes advantage of the primary amine functional group to react with a heterobifunctional ligand sulfo-SMCC which has a reactive sulfo-NHS group on one end which links to the primary amine, and a maleimide group on the other end. The maleimide group is reactive towards thiol groups allowing conjugation with thiol-functionalized biomolecules. You can also utilize enzymatic bioconjugation methods for site-specific reactions to nanoparticles.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Method for Thiol Conjugation to Gold Nanoparticles
One study takes advantage of gold’s high affinity for thiol groups by first reacting succinimidyl propionyl poly(ethylene glycol) (PEG) disulfide with the primary amine of an antibody. The PEG/antibody complex has a disulfide bond which readily reacts with the gold nanoparticles, forming Au-S bonds and a bioconjugate system. This system was used for early-stage cancer diagnosis and thermal therapy of tumors.
Bioconjugation of Iron Nanoparticles
Functional Groups on Iron Nanoparticles
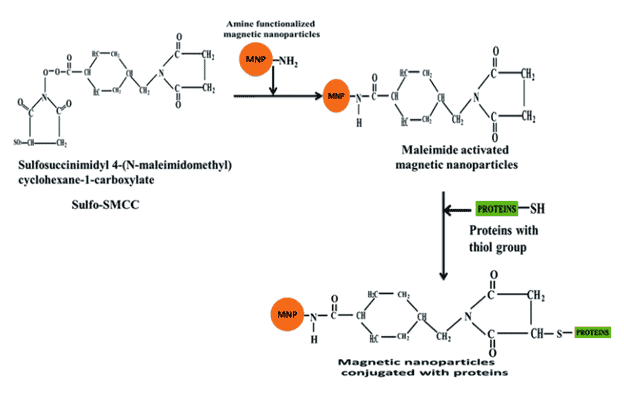
Common functional groups on iron nanoparticles include amines and carboxylic acids. Use Sulfo-SMCC to first react with amine groups on nanoparticles and then utilize the maleimide group to react with other thiol-containing compounds.
Amine Functional Groups for Bioconjugation to Iron Nanoparticles
Amine groups can be introduced onto the surface of iron nanoparticles using salinization, chemisorption, carbodiimide activation, and many other methods. Like gold nanoparticles the positive charge on the amine group allows for bonding with negatively charged oligonucleotides as well as many other surface functionalizations with both homo and heterobifunctional linkers.
Amine functionalized nanoparticles are great for the separation of DNA. The super magnetic nanoparticles can bind with DNA and a magnet can be used to capture the conjugated particles while the rest of the solution is decanted and rinsed. Learn more about DNA bioconjugation techniques in our article.
Method for Amine Conjugation on Iron Nanoparticles
Amine conjugation can be done using both physical interaction (ionic, hydrophilic/phobic, and affinity interaction) as well as covalent chemistry. Direct conjugation is a facile method and can be done with amine, sulfhydryl, and aldehyde groups.
Amine functionalization however does not suffer the problems the other mentioned functional groups have with intercalating and cross linking. You can learn more about the problems with bioconjugation and how to choose bifunctional crosslinkers in our articles.
One study accomplished protein coupling to the magnetic particles by first reacting Sulfosuccinimidyl-(N-maleimidomethyl) cyclohexane-1-carboxylate (Sulfo-SMCC) with the primary amine of the magnetic nanoparticles. The maleimide complex then reacts with a thiol-containing protein to form the magnetic nanoparticle conjugate.
