Post translational modification (PTM) is the process of functionalizing proteins after they have been translated in the ribosome. This is a common process that occurs naturally in the body and serves many purposes for applications such as protein folding or modification of protein activity. Synthetic post translational modification is a common topic of research utilized in biomedicine for research in heart disease, cancer, and many illnesses. However, site-specific modification of proteins is challenging without the use of enzymatic reactions. Methods have been developed to achieve protein bioconjugation with amino acids such as cysteine, as well as oligonucleotides for a multitude of applications, but many result in protein unwinding, undesired modifications, and poor biconjugate stability. In this article we discuss the use of palladium in bioconjugation of proteins and DNA site-specifically.
The use of Palladium in bioconjugation of proteins and DNA enables site-specific modification with fast kinetics in mild conditions. Palladium catalysts can be used to introduce aryl-groups onto thiolate functional groups on cysteines and to add acyl groups to DNA and RNA oligos.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
A Method Using Palladium in Bioconjugation of a Protein Via Cysteine Amino Acids
Although there are already many great applications and synthesis routes for producing protein bioconjugates, many of these reactions require unnatural amino acids and high concentrations. If these unnatural amino acids are not used, the end result is non-specific. Thus, to overcome these limitations, scientists have begun developing reactions using palladium complexes created from oxidative addition of aryl halides for the addition of aryl groups to the cysteine residue of protein polypeptide chains. This aryl group can then be reacted to form many other bioconjugates (see ref). In this section we discuss a general method for using palladium in bioconjugation of a protein. For more details, refer to Vinogradova et al.
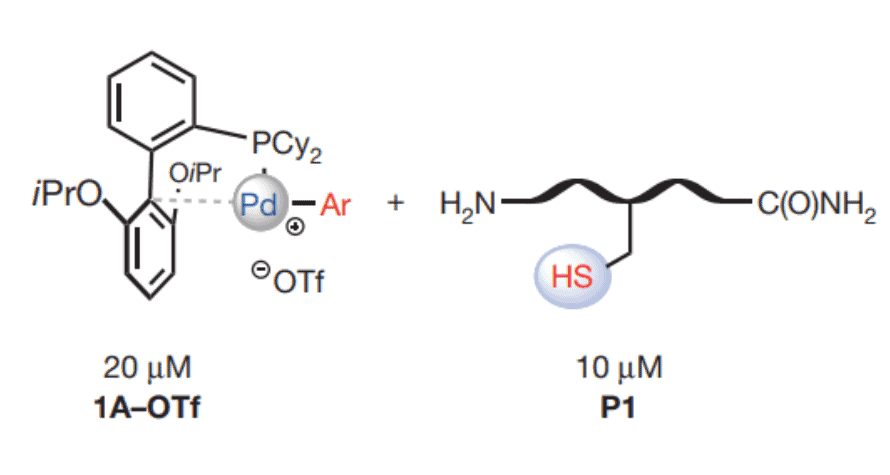
Step 1. Conjugation of Aryl Ligand to Cysteine Residue
First the palladium-tolyl complex (1A-OTf shown in figure above) was dispersed in solution with trifluoromethanesulfonate to act as a counterion. This created a stable solution and allows for good dispersion of the palladium reagent. Here 2-dicyclohexylphosphino-2’, 6’-diisopropoxybiphenyl (RuPhos) was the ligand used for conjugation of the protein.
Next, a model peptide was added to the solution and allowed to incubate for 5 min at room temperature. Here the ligand is transferred to the thiolate group of the cysteine residue forming the site-specific conjugate pair.
FYI: An alternative method to attach site-specifically onto proteins is to utilize enzymatic bioconjugation techniques
Step 2. Conjugation of Aryl Group
Finally, the aryl group can react with fluorescent probes and many other reactive species to create bioconjugates for many different applications.
A Method Using Palladium in Bioconjugation of an Oligonucleotide
Similar to the bioconjugation of proteins, oligonucleotide conjugation also has many challenges due to the large number of functional groups available on DNA and RNA molecules. Palladium has also been used to engineer site specific conjugation of oligonucleotides for many applications. In this section we discuss a general method for using palladium in bioconjugation of oligonucleotides. For more details, refer to Jbara et al..
Step 1. Acylation of Oligonucleotide
Bifunctional-palladium(II) reagents were used with amine-modified oligonucleotides for the acylation of the oligonucleotide to generate palladium(II)-oxidative addition complexes (OACs). Because of the high nucleophilicity of the palladium complex these reactions are highly selective. If you are looking for another method to react with amine-modified oligos, take a look at our article on NHS ester bioconjugation techniques. We’ve also discussed different lysine conjugation protocols in this article.
Step 2. Thiol Targeted Bioconjugation
The OACs are then reacted with the thiolate of a peptide or some other thiol containing compound to form the conjugate pair. These reactions can be performed in mild aqueous solutions and lead to highly selective and mild reaction processes.
Applications of Cysteine Bioconjugation
Cysteine is a thiol containing amino acid present in the human body in polypeptide chains. Cysteine residues present in protein polypeptide chains have become a common target site for controlled bioconjugation of proteins due to high nucleophilicity of the thiol group, diverse number of possible reactions, and its low abundance in proteins. These characteristics make cysteine the ideal target for stereospecific Michael addition reactions to maleimides and substitution reactions with alkyl halides. Methionine conjugation and tryptophan conjugation are other techniques that can be used to target low abundance amino acids.
Applications of cysteine bioconjugation include Fluorescence Resonance Energy Transfer (FRET) Spectroscopy, antigen detection, site specific delivery of cancer therapies, and bio and cellular imaging.
Related articles:
- Azide bioconjugation is another popular technique for bioconjugation to proteins
FRET Spectroscopy Antigen Detection
FRET Spectroscopy takes advantage of donor and acceptor pairs to create fluorescence or change the fluorescent emission of a probe. By using FRET it is possible to detect reactions, changes to conformations, and specific site binding interactions. One study took advantage of FRET for detection of HIV. This was achieved by bioconjugation of Cy3 dye to the thiolate of the cysteine residue on the antigen protein. After site specific modification, binding of HIV antibodies led to conformational changes allowing for resonance and enhancing the fluorescence of the dye shown in the figure below.
Cancer Treatment
Cysteine bioconjugation is also used for cancer treatment by site specific bioconjugation of platinum moieties via maleimide reactions. These systems can be made from many different types of platinum compounds conjugated onto proteins or peptides. These conjugate systems are absorbed by the cancerous cell wall and once inside, the conjugate molecule undergoes hydrolysis, transforming platinum to a positively charged molecule capable of binding to DNA. Once bound to the DNA the platinum can cause the cell to undergo necrosis or apoptosis. Cysteine bioconjugation of radiopharmaceuticals is another approach to develop anti-cancer therapeutics.
Bio and Cellular Imaging
Bio and cellular imaging is another area of great interest in modern research. Bioconjugation of the cysteine residue is often used for site specific bonding of quantum dots and other nanoparticles onto protein surfaces to produce stable colloids for bio imaging. The particles are then delivered to cells or undergo different types of interactions to be detected by analytical methods such as Surface Plasmon Resonance, Fluorescence Spectroscopy, and confocal microscopy. The figure below shows the results from one study where quantum dots conjugated onto cysteine residues of goat antibodies were used for fluorescent detection of h-IgG, a protein used for proof of concept experimentation.
Applications of Oligonucleotide Bioconjugation
Oligonucleotides are short DNA and RNA oligomers that have become an integral part of modern research. They are typically laboratory synthesized and have many applications including fluorescence spectroscopy, treatment of neurological diseases, and protein sensing. We’ve covered a variety of DNA bioconjugation techniques in another article.
Oligonucleotides can be selectively conjugated using palladium-based catalysts. Applications of oligonucleotide bioconjugation include fluorescence spectroscopy, treatment of neurological diseases, and protein sensing.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Fluorescence Spectroscopy
Oligonucleotides can be labeled with fluorescent probes and used for fluorescent bio sensing and DNA sequencing. One Common configuration is depicted in the figure below. The electron donor and acceptor pairs are bound to the tails of the oligonucleotide which interact due to their close spatial arrangement causing quenching of the fluorescent emission. However as the oligonucleotide interacts with its target the acceptor donor pair are no longer close enough for resonant interactions and the molecule no longer experiences fluorescent quenching.
Illustration of a fluorescently labeled nucleotide used for detection of (A) specific genes, (B) triplex formation, and (C) protein-DNA interactions, via FRET spectroscopy. Source: Chemical Society Reviews.
Treatment of Neurologic Diseases
Another possible use of oligonucleotides is for the treatment of neurologic diseases. Oligonucleotides are conjugated to peptides to efficiently deliver them to target cells. Peptides are used as a transport mechanism because of their ability to move across cell membranes without receptor-mediated endocytosis. Once inside the cell the oligonucleotides can help to treat neurological disorders by either changing, removing, or restoring proteins causing certain gene expressions leading to the disorder. The figure below shows how antisense oligonucleotides (ASOs) can accomplish these tasks.
Protein Sensing
Protein sensing, specifically sensing of lectins, is another topic of interest in the medical world. Lectins are proteins that are present in nearly all organisms and are characterized by their specific recognition of carbohydrates. By conjugating carbohydrates to oligonucleotides, protein specific and efficient delivery of the oligonucleotides to the lectins can be achieved (see figure below).
