Radiopharmaceuticals are active biological molecules that are tagged by radionuclides. They are used for diagnostic imaging and for therapeutic applications. Typically, radiotracers utilized on radiopharmaceuticals emit ionizing radiation in small doses and can be detected using a PET or SPECT instrument. In this article we will discuss different bioconjugation methods for radiopharmaceutical chemistry, applications of these conjugates, and protocols for attaching radioisotopes onto biomolecules.
Bioconjugation methods for radiopharmaceutical chemistry typically involve the use of chelation using p-Isothiocyanatobenzyl (DOTA) or deferoxamine, pegylation, or bioorthogonal reactions with click-chemistry or tetrazine ligation chemistry.
Applications of Radiopharmaceutical Chemistry
There are numerous applications of radiopharmaceutical chemistry because radiotracers can be detected at very low concentrations. Typical applications include diagnostic imaging, therapeutics, and imaging of tissues that are hard to access like those in the brain and nervous system.
Applications of radiopharmaceutical chemistry include diagnostic imaging using SPECT or PET, therapeutics which use α or β emitters, and imaging of tissues that are hard to access such as neuronal and nervous system tissues.
Application 1. Diagnostic Imaging
Radio-diagnostic imaging is a non-penetrative, quick method, in which a radiotracer is perfused into the human body at very low concentrations (nM to pM). At such low concentrations, there are no pharmacological effects. However the use of radiotracers enables us to identify early diseases, evaluate physiology, and monitor medication in real-time. Diagnostic probes are frequently attached to the C-terminus of antibodies.
By utilizing radio-diagnostics, it’s possible to tailor therapies to the needs of patients. It’s now common practice to use radio-diagnostics for personalized medicine.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Two methods for imaging radio-diagnostics include SPECT and PET. These are highly sensitive molecular techniques that have the ability to detect pathologies early. They can also be utilized for real time monitoring of biochemical processes including tumor growth and hypoxia, proliferation, angiogenesis, multi-drug resistance etc. Furthermore, PET imaging can also provide information on toxicity, kinetics, and drug distribution within the body.
In diagnostic nuclear medicine, technetium-99m is the most often used radioisotope. It can be linked to a wide range of molecules and allows doctors to detect a wide range of illnesses including cancers. For example, in this paper, the authors used technetium-99m-MDP (methylene diphosphonate) to detect bone metastasis. .
Application 2. Therapeutics
Radiopharmaceuticals are novel therapeutics to treat cancers. Typically, radiopharmaceuticals that are used in therapeutics contain an α or β emitter and auger electrons that can kill local cancer cells – ie. the therapeutic radiopharmaceuticals are delivered locally to tumors in high doses and are able to kill cells with ionizing radiation.
Radiopharmaceutical emitters with a high linear energy transfer (LET) decrease their energy by emitting α particles. These particles, in turn, kill tumors and metastases which are a short distance away (a few cell diameters).
For larger tumors, low LET emissions provided by β emitters are utilized because they can penetrate deeper (40 to 60 times the diameter of cells).
For radiopharmaceutical therapeutics to be effective, scientists conjugate them in a way to maximize tumor cell uptake, and residence time while also maximizing the rate of clearance from healthy cells. Typically, the radiation from radiopharmaceuticals is delivered systemically or locoregionally, similar to chemotherapy, rather than from outside the body, as is the case with radiotherapy. We’ve discussed bioconjugation and cellular uptake in more detail in another article.
Application 3. Neuroscience, Neurology and Psychiatry
Over the last 30 years, researchers have devoted a lot of effort to understand the relationship between brain behavior, chemistry, and disease. Although considerable development has been made, only a few hundred metabolic processes and neurotransmitters that operate within the brain and make it function can be quantified and imaged with high specificity.
Currently, ongoing research with radiopharmaceuticals is being utilized to identify molecular abnormalities caused by neurodegenerative disorders like Parkinson’s, Alzheimer’s, and psychiatric illnesses like depression and schizophrenia.
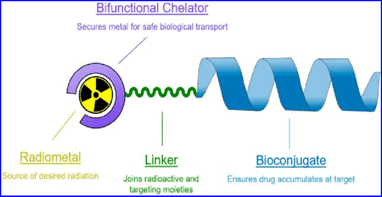
Bioconjugation Methods for Radiopharmaceutical Chemistry – An Overview
Bioconjugation refers to the biochemical or chemical processes used to produce a stable linkage between a biomolecule and a second biological or chemical moiety. In this description, biomolecules represent relatively large, nature-inspired or naturally occurring molecules that are able to perform different biological functions. They are classified into four categories: polypeptides such as antibodies and peptides, polysaccharides, nucleic acids such as DNA and RNA, and lipids.
In bioconjugation, a primary biomolecule can be attached to a secondary biomolecule or to a different chemical entity like a drug, toxin, imaging reporter like a radionuclide, or a polymer like PEG. Below we’ll discuss different bioconjugation methods for radiopharmaceutical chemistry.
Related articles:
- Looking for methods to react with specific amino acids, for example tyrosine bioconjugation? Take a look at our other article.
Method 1. Chelation Using Dota (P-isothiocyanatobenzyl)
Chelation is a method in which an organic moiety acts as ligand that binds to a metal ion via two or more coordination bonds. These multiple-bonded or polydentate ligands cluster around a single central atom. These ligands are also known as chelators, chelants, sequestering agents or chelating agents.
DOTA (p-Isothiocyanatobenzyl) peptides form metal complexes and are commonly used in targeted therapeutics, MRI contrast agents, and radiopharmaceuticals. DOTA can be conjugated to antibodies or to proteins via their lysine side chains (which contain primary amine groups). Lysine groups are typically found on protein surfaces and can be functionalized easily because of their hydrophilic and polar nature. You can learn more about lysine conjugation and other protein conjugation techniques here.
To react with lysines, dissolve DOTA in water and react it with the lysine side chains using amine chemistry in moderately alkaline solutions (pH 8-9). You might find this article about challenges and techniques for bioconjugation in water interesting as a follow-up to this section.
Method 2. PEGylation
PEGylation is the process of bioconjugation of PEG (polyethyleneglycol) polymers to biomolecules via covalent linkages. PEG is biocompatible, non-antigenic, non-immunogenic, and non-toxic (source). It’s also water-soluble and easily soluble in other organic solvents like acetone, alcohols, and chloroform. In solutions, PEG has high mobility and it is easy to conjugate to the molecules. PEG is one of the few synthetic polymers that are GRAS (generally recognized as safe) for internal administration by the FDA.
PEGylation of a molecule results in modification of the molecule’s physicochemical properties. It will increase the size (due to the additional PEG polymers) and due to PEG’s masking effect it will also increase the retention of the biomolecule or therapeutic agent within the body. PEG will also enable the biomolecule to cross cell membranes through endocytosis to reach intracellular targets. You can learn more about the effects of PEGylation here and we’ve discussed bioconjugation to pegylated nanoparticles (which could be used to deliver radiopharmaceuticals) here.
Alternatively, PEGylation can involve the creation of liposomes which can carry radiopharmaceuticals. For example, in this paper, PEGylated liposomes were used to encapsulate rhenium-188. Release of rhenium-188 inhibited the proliferation of cancer cells in vivo and repeated administration of rhenium-188 conjugated radiopharmaceuticals increased efficacy.
Polymers can be conjugated to proteins and antibodies using a range of functional groups such as amines, carboxyls, or thiols. Explore conjugation kits for polymers, proteins, and antibodies, here.
Method 3. Bioconjugation Using Bioorthogonal Reactions
Bioorthogonal reactions are chemical reactions that don’t interfere or interact with biomolecules in living systems because they use unnatural functional groups. Ideal bioorthogonal reactions utilize components that are non-toxic, inert to biological systems, highly reactive, and highly selective with one another when present in biological systems.
Bioconjugation of radiolabels through bioorthogonal reactions basically involves two steps:
- First, a reactive component is introduced into a biomolecule biochemically or chemically.
- Then a radiolabel is attached to the reactive component via a bioorthogonal reaction.
In both clinical and preclinical research, fluorine-18 is the most commonly utilised radiolabel and it is used in PET. Other examples are Yttrium-86, Zirconium-89, Iodine-124, Copper-64, Rubidium-82 etc.
Several bioorthogonal reactions have been developed for bioconjugation. These include click reactions, staudinger ligation, photo-click reactions and tetrazine ligation. We’ve covered protocols for several orthogonal bioconjugation techniques here. Additionally, you might consider electrochemical bioconjugation techniques which are more site-specific and bioorthogonal.
Step by Step Methods for Bioconjugation of Radiolabels
Method 1. Isolation and Synthesis of an 18F-Labeled Bovine Serum Albumin Derivative
The method presented below is a high-level overview of how to attach 18F to BSA protein. For more details, take a look at the original article. Tryptophan bioconjugation and cysteine bioconjugation can give you more site-selective methods for attaching 18F radiolabels.
Step 1. Prepare 18F Azide
0.1ml azide (18F) 120.1 mL, 1.1 Gbq, 30 mCi was added in 2mg 10.10 μmol sodium ascorbate solution while stirring.
Step 2. Add in BSA
2 mg, 8.01 μmol copper (II) sulfate pentahydrate, 1.7 mg, 3.9 μmol 3,3′,3″-((nitrilotris(methylene))tris(1H-1,2,3-triazole-4,1- diyl))tris(propan-1-ol) were dissolved in 0.25ml of 1X PBS buffer and 0.6ml of 1mg/ml solution of BSA was added.
This solution was stirred gently at 40 ºC for 10 minutes. This revealed that 81% of radiochemicals converted to 18F-labeled BSA.
Step 3. Purify 18F-labeled BSA
This mixture was purified by using a PD-10 column. It was eluted in 6 separate vials which contained 1ml of 1X PBS per sample.
Method 2. Preparation of 89Zr-labeled Deferoxamine-NCS and J591 Monoclonal Antibody
Below we describe the general scheme for preparation of a radiolabeled antibody J591. For a more detailed protocol, please refer to the original research article.
Step 1. Conjugate Deferoxamine-NCS Chelator to J591 Antibody
First, prepare a 2 to 5mg/ml solution of J591 in 1ml of 0.5M HEPES buffer (pH 7.4) or PBS buffer (pH 7.4) in a 1.7ml microcentrifuge tube.
In dry DMSO dissolve DFO-NCS at concentration 5 to 10mM (3.8-7.6 mg/ml). Vortex or sonicate the solution to completely dissolve the solution.
To adjust pH of J591 soln. 8.8 to 9.0 small aliquots (less than 10 µl) of 0.1 M Na2CO3 is used.
When the antibody solution gets the correct pH, add DFO-NCS solution corresponding with 3 to 4 fold molar excess of (bifunctional) chelator.
Incubate the reaction at 37 ºC for 30 minutes on agitating (heating block) at 350 rpm.
After 1 hour incubation the purify the immunoconjugate by size exclusion desalting column. 0.5M HEPES buffer (pH 7.4) acts as eluent.
Using UV spectrophotometer determine the concentration of J591-DFO construct.
Store this solution at -20 ºC in the dark.
Step 2. Radiolabel J591-DFO with 89Zr
Prepare 0.5 to 2.0 mg solution of J591-DFO in 0.5M of 200µl HEPES buffer (pH 7.5).
Pipette 89Zr4+ stock solution corresponds to 1.0-6.0 mCi in a microcentrifuge tube of 2ml plastic screw cap. Adjust volume by 1M oxalic acid to 300µl.
Adjust 89Zr4+ pH to 6.8-7.5 by using 1M Na2CO3.
Add 89Zr4+ solution to J591-DFO solution (prepared earlier).
Check the pH to ensure the desired pH range.
Incubate the (radiolabeling) reaction for 60 minutes at room temperature on agitating (heating block) at 350 rpm.
By using radio TLC, determine the radiolabeling yield after 60 minutes of incubation.
If the yield of radiolabeling is sufficient, quench reaction with 5 µl of 50 mM DTPA (pH 5.5).
Purify the immunoconjugate by size exclusion desalting column by using eluent sterile saline 0.9% with 5 mg/ml gentisic acid or 0.25 M sodium acetate (pH 5.5) with 5 mg/ml gentisic acid.89Zr-DFO-J591 radiochemical purity can be determined by using radio- TLC.
