Tryptophan, sometimes abbreviated to Trp or W, is an essential amino acid, meaning that humans cannot synthesize it in the body. Its side-chain group is an indole which makes it an aromatic amino acid. There are many proteins that use tryptophan in their structure and these terminal tryptophan groups can be used in bioconjugation reactions to make fascinating bioconjugate molecules with a variety of applications. In this article, we explore a variety of tryptophan bioconjugation methods.
Tryptophan bioconjugation methods typically use the following methods: reaction with the indole ring C2, reaction with the indole amine groups, or reaction with the indole aromatic ring.
Related articles:
- Are you attaching your protein to an oligonucleotide or to DNA? Read our article on DNA conjugation techniques.
What is Bioconjugation?
Bioconjugation is the process by which scientists create links between molecules, where at least one of those molecules is biological. The biological molecules used can range from small simple molecules such as carbohydrates to larger, more complicated biological molecules such as proteins and DNA. Bioconjugate molecules have been used in a variety of applications ranging from new pharmaceuticals, to biosensors, to novel biomaterials.
Bioconjugation is a synthetic strategy used to create chemical bonds between two or more molecules of which at least one is biological.
However, bioconjugation chemistry is not without its challenges. Chemical reactions can have various effects on biomolecules. For example, the shape of proteins can be affected by the electronic and steric effects on the conjugates molecule or the conditions of the reaction can denature an enzyme, rendering it useless. You can learn more about the challenges with bioconjugation in our sister article.
Common Protein Amino Acids Utilized for Bioconjugation Reactions
There are many amino acids found in proteins that can be utilized in bioconjugation reactions. The specificity of which will depend on the type of chemistry used and the structure of the protein. Here are some of the most common amino acids in proteins used for bioconjugation:
Common protein amino acids utilized for bioconjugation reactions include cysteine residues, lysine residues, and tyrosine residues.
1. Cysteine residues
Cysteine is a sulfur-containing amino acid that is semi-essential in the human body. It’s an excellent protein to use because of the availability of the thiol functional group. Thiols can be used to produce a variety of conjugate bonds with other molecules. Cysteine rarely appears on the protein surface making it an excellent target for bioconjugation and modifications. There are a variety of specific and non-specific bioconjugation methods for cysteine available to meet your needs. We’ve discuss palladium bioconjugation to site-specifically attach cysteines in another article.
Thiols react readily and can be used for conjugation reactions. Papyrus Bio has a range of thiol-based conjugation kits. Explore thiol conjugation kits here.
2. Lysine residues
Lysine is another abundant amino acid found in proteins. It has a lysyl side chain containing 4 carbons and an amine group. The free amine group found in lysines structure makes an excellent target for bioconjugation reactions because, like thiols, amines have a variety of bonds that can be made. Lysine is also excellent because it can be modified on proteins under relatively mild conditions. Just like cysteine, there are a variety of specific and non-specific methods available for utilizing lysine residues in bioconjugation reactions. Be careful about side-reactions with lysine groups when trying N-terminal bioconjugation of proteins.
3. Tyrosine residues
While traditional cysteine and lysine bioconjugation strategies are commonly used and have extensive research available, they aren’t always a compatible bioconjugation target. With that in mind, tyrosine is a less explored alternative that is becoming a popular alternative to the more commonly used strategies. Tyrosine is rarely surface exposed in proteins, making it an excellent target for site-specific modifications. It’s also less reactive and forms more bonds that are more stable than those commonly used for cysteine and lysine conjugation.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Overview of Tryptophan Conjugation Chemistry
The indole moiety in tryptophan’s structure opens up many possibilities for bioconjugation strategies. Furthermore, tryptophan is an excellent bioconjugation target because it has low surface abundance which allows for increased specificity. Generally, the conjugation reaction takes place at the active carbon on the double bond of the indole ring (C2). For example, in this paper, the authors created tryptophan-folate conjugates at that carbon.
Create conjugate bonds on the active carbon on the indole ring (C2) of tryptophan. This is the most common tryptophan bioconjugation chemistry reaction. Conjugate bonds can also be formed at the indole nitrogen and on the aromatic ring.
However, there are other parts of the tryptophan structure that can be used for bioconjugation reactions. In this paper, the authors found that the tryptophan-gold nanoparticle bioconjugates were primarily bonded on the indole amino groups and the carboxylic acid groups. The authors used the tryptophan-gold nanoparticle conjugates for deep UV imaging of microbial cells.
Conjugation reactions can also be performed on the aromatic ring of the indole group on tryptophan. This is because of the electronic effects of the nitrogen on the reactivity of the aromatic ring. In this paper, the authors selectively modified tryptophan residues on proteins using a biomimetic electron transfer process. The authors used this method to selectively label the tryptophan group on the protein.
We discuss other methods for protein-nanoparticle conjugation in another article.
Tryptophan Bioconjugation Methods
There are many different bioconjugation methods available that utilize tryptophan residues to form the conjugate bond.
Tryptophan bioconjugation methods include ABNO based reactions, UV crosslinking, photocatalytic conjugation, and treatment with strong bases like KOH.
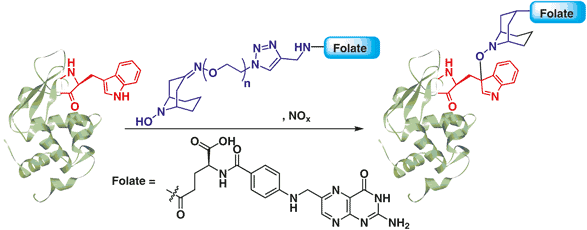
Method 1. Conjugation of Protein-Trp to a Vitamin Using ABNO-PEGn-Folate
The authors of this paper used a tryptophan-selective reaction to conjugate folate to an antibody. Folate is a well-known ligand of cancer cells. The authors intended for this conjugate to be used to add extra functions to antibodies while preserving the function of the folate. This, in turn, could then be used for drug delivery for cancer imaging purposes. The authors used an ABNO-PEG-Folate compound that reacted with the tryptophan groups on the protein in solution. The reaction conditions were mild enough that it only needed gentle stirring at room temperature for 30 minutes for the reaction to proceed. Even without additional heating, the reaction still had high tryptophan sensitivity and high homogeneity.
While not tryptophan specific, you might enjoy reading our article on protein-protein conjugation techniques.
Method 2. Labeling Protein-trp Using 2,2,2-trichloroethanol
Because of its relatively low abundance and limited surface presence, tryptophan groups are an excellent target for selective labeling of proteins. Selectively labeled proteins can be used in a variety of ways to study the structure of proteins, the use of proteins in cells and the body, and for biological imaging. In this paper, the authors labeled tryptophan groups on proteins with 2,2,2-trichloroethanol for quantification and visualization with UV light. The authors noted the high sensitivity of their method.
For this labeling, the authors reacted tryptophan with the 2,2,2-trichloroethanol in an aqueous solvent. The reaction was catalyzed under a 15 W UV light for 15 minutes. The reaction’s progress could be tracked by measuring fluorescence emissions at 450 nm and 310 nm.
Method 3. Conjugation Of Tryptophan To A Nanoparticle
In this paper, the authors used tryptophan-coated gold nanoparticle conjugates to inhibit the amyloid aggregation of insulin. This was interesting because tryptophan on its own was unable to inhibit insulin aggregation. The tryptophan-gold nanoparticle bioconjugates were synthesized by boiling aqueous solutions of KOH and tryptophan. Gold metal ions were added to the boiling solution and allowed to form nanoparticles.
Attaching proteins to carbon nanotubes can increase circulation time and tumor targeting. Learn more about how to do this in our other article.
Method 4. Photocatalytic Conjugation Of Tryptophan to A Michael Acceptor
In 2018, the authors of this paper managed to create a photocatalytic conjugation technique that allowed for the tryptophan groups on a peptide to be conjugated to any Michael acceptor. The authors managed to use both glucagon and GLP-1 amide at the tryptophan beta position. Interestingly, the reaction was achieved in an integrated photoreactor using 450 nm light in the presence of an iridium-based catalyst in DMF.
Electrochemical Tryptophan-selective Bioconjugation
One exciting novel method for the selective bioconjugation of tryptophan groups was outlined in 2019 by the authors of this paper. They were the first researchers to successfully use an electrochemically promoted method to selectively bioconjugate proteins in neutral aqueous media. They used lysozyme and bovine serum albumin. The method is particularly interesting because it uses metal-free, mild conditions. The authors hoped that this method would be applicable for cleaner scalable bioconjugation reactions as the ketone group on the TEMPO can be further manipulated for bioconjugation reactions. An example of using ABNO chemistry for tryptophan-selective bioconjugation can be found in the following section.
Here is an outline of how they did it:
Step 1. Prepare a mixture of your protein, keto-ABNO, and 4-oxo-TEMPO
These compounds should be dissolved in 0.1 M LiClO4 at room temperature in air.
Step 2. Electrolyse the reaction mixture with an electric charge of 0.9 V
Electrolyse the mixture using a graphite (+) – platinum (-) undivided cell at 0.9 V.
Step 3. Stir the mixture for 1 to 3 hours while the current is active
The reaction will need around 1 to 3 hours to go to completion. The authors used mass spectrometry to measure the progress of the reaction.
Step 4. Isolate your protein conjugate
In many cases, the author reported a nearly quantitative yield. Protein chromatography is an excellent method for isolating proteins and protein conjugates.
Using Tryptophan-Selective ABNO Chemistry for Bioconjugation
After successfully developing their technique for tryptophan specific bioconjugation reactions, the authors were able to apply ABNO chemistry to conjugate folic acid to a monoclonal antibody in this paper. The folic acid-antibody conjugate showed activity against folate receptor-positive cancer cells, suggesting potential uses for fluorescence imaging and anticancer drug delivery.
In their work, the authors modified the linker lengths of the conjugates to study their effect on their binding to cancer cells. They found that the fluorescence was increased with the length of the PEG linker in the structure suggesting a stronger binding.
Site-Selective Bioconjugation of Tryptophans Using Fluoroalkyl Radicals
One novel technique for the site-selective bioconjugation of tryptophans involves the use of fluoroalkylated cyclic iodanes, as outlined in this paper. The results of the study were patented and used in the CF Link bioconjugation agents offered by CF PLUS Chemicals. As part of their work, the authors were able to fluoroalkylate a range of proteins, including bradykinin, somatostatin insulin, myoglobin, ubiquitin, and carbonic anhydrase. The manufacturers have suggested several applications including attaching toxic/radioactive payloads, fluorescent dyes, stabilizers, and various large and small molecules.
The method offers several benefits including controlled site-specific conjugation at the tryptophan indole ring. It also offers improved stability compared to maleimide conjugation reactions. The reaction is also transition metal-free, which offers benefits over alternative methods. However, the method does present some challenges due to the requirement to synthesize fluorinated precursor compounds. Fluorine chemistry requires additional safety and regulatory requirements in most labs. An alternative method for site-selective conjugation to proteins is to use transglutaminase bioconjugation, we’ve written about this method in detail in our article. We’ve also covered bioconjugation to metal-complexes in another article.
Here’s a rough step by step guide to how the CF link reagents work:
Step 1. Activate the hypervalent-iodine reagent
This is performed by adding sodium ascorbate in an aqueous buffer solution.
Step 2. Give the reagent time to activate
The fluoroalkylated cyclic iodane compounds will decompose in solution to form a fluoroalkyl radical.
Step 3. The radical functionalizes the tryptophan residues on the target molecule
Fluoroalkyl radicals in solution attack solvent-accessible tryptophan residues on the target molecule at the two position on the aromatic ring.
Step 4. The payload molecule is added and bioconjugated via click chemistry
The bioconjugate is rapidly formed in solution through a click chemistry reaction that occurs upon the addition of the payload molecule. You can learn more about ‘click’ azide bioconjugation chemistry here.
