Bioconjugation techniques are a powerful tool in the development of new biotechnologies such as biosensors, advanced drug-delivery systems, and more. However, given the complexity of bioconjugation chemistry, it’s no surprise that many challenges need to be overcome when developing bioconjugation techniques. In this article, we discuss some of the common bioconjugation problems and their solutions.
Common bioconjugation problems include lack of site-specificity, biomolecule degradation, and unstable bioconjugates. Common solutions include using activating biomolecules to open up specific sites, using less-harsh reagents, and storing bioconjugates in cool conditions.
What is Bioconjugation?
When you need to combine different biomolecules you need to utilize bioconjugation techniques.
Bioconjugation is the formation of new molecules by combining two or more different molecules, one or more of which is a biomolecule.
Commonly used biomolecules for bioconjugation reactions include proteins, DNA, antibodies, and more. These biomolecules can be attached to a range of molecules such as plastics, nanoparticles, metals, drugs, fluorescent molecules, and many others.
The applications of bioconjugates are wide and varied. Bioconjugates are often used in advanced drug-delivery systems and therapies, novel biosensors used in medical science, biological and medical imaging, and more.
Related articles:
- Learn more about bioconjugation to nanoparticles in our article.
- We’ve discussed bioconjugation to metal complexes in a related article
Bioconjugation Problems Related to Proteins
Proteins are often used in bioconjugation chemistry. There are many reasons for this including biological monitoring by labelling live proteins, creating protein biosensors, stabilizing proteins in protein therapies, and imaging. Some proteins are relatively easy to work with and can be manipulated for bioconjugation chemistry on a larger-scale. However, others may prove difficult and several problems might need to be faced.
Bioconjugation problems related to proteins include lack of access to the reaction site, lack of site-specificity, and a lack of bioconjugation techniques for different amino acids.
Lack Of Access To The Reactive Site
Often, when dealing with native proteins, the desired reactive groups are limited or inaccessible for reactions due to the structure and folding of the protein. This can lead to poor yields of the bioconjugate product or, worse, a failure of the reaction. This problem is arguably the most difficult to overcome if your goal is to retain the native nature of the protein. Thus, creative solutions to the problem are required.
Here are some possible solutions:
- Find an alternative reaction site: This seems obvious, but if you want your protein to remain in its native state for your desired application, then you’re going to have to look for alternative reaction sites.
- Directly modify the protein: If modifying the protein is acceptable for your goals, you can modify the protein to give you better access to your target reactive site (source).
- Modify the protein with genetic manipulation: If you have access to the tools required to genetically modify your target protein, you can make adjustments to the structure of the protein that makes your target reactive site more accessible (source). However, this kind of modification cannot be used in certain cases. For example, you cannot modify cysteine residues on a protein that has Cys residues playing a crucial role in its activity.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Site specificity
In some cases, your bioconjugation may lack site-specificity. This means that you’re not able to only target the specific reaction site you desire for your bioconjugation reaction. Here are some ways to overcome this problem:
- Incorporate an unnatural amino-acid into the structure: If you want to be sure your bioconjugation reaction specifically targets the site you want, why not choose the site itself? By incorporating an unnatural amino-acid into the structure and synthesizing your target protein with it, you can tailor your reaction to conjugate at the site you’ve chosen (source). This strategy can be used for site-specific N-terminal bioconjugation to proteins.
- Use a catalyst to promote a site-specific reaction: The use of the right kind of catalyst can let you use a site-specific reaction for your bioconjugation. There are a variety of catalysts available that might be able to help. For example, the authors of this paper used enzymes to trigger site-specific protein-protein bioconjugation.
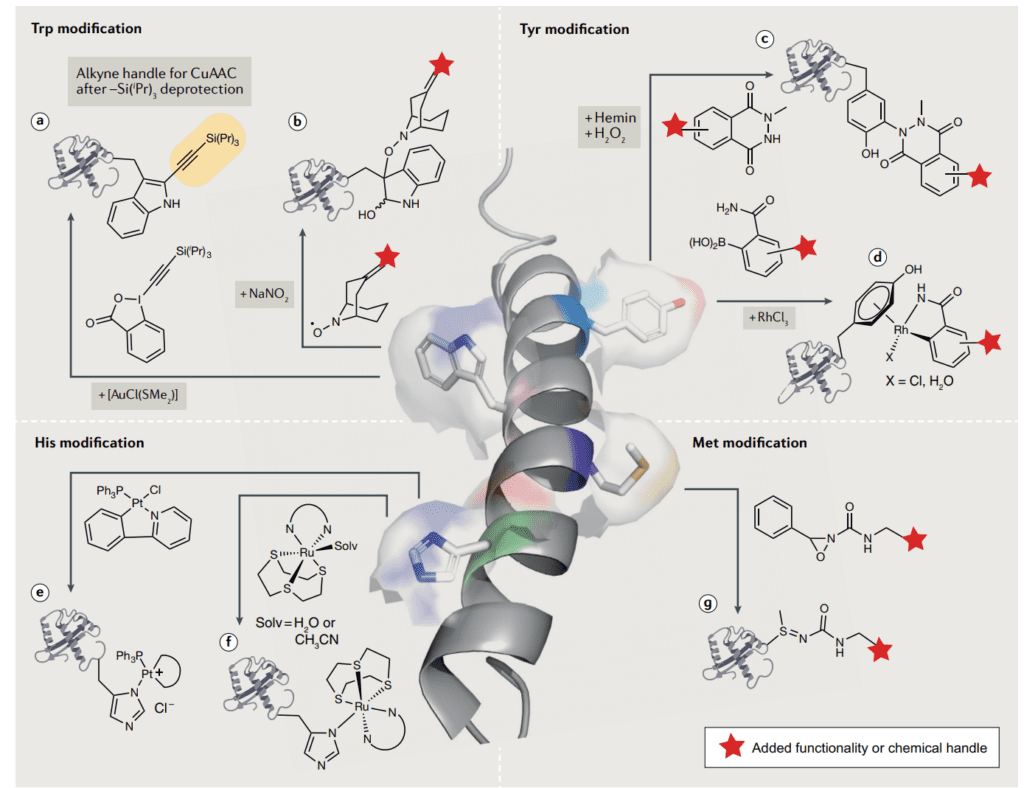
Lack of bioconjugation techniques for different amino acids
Sadly, there isn’t always a bioconjugation technique available for your specific purpose. No-one may have performed the specific reaction that you’re interested in or used the amino acid that you have in mind. For example, tyrosine is only recently emerging as a bioconjugation target. We’ve described some methods for tryptophan bioconjugation and methionine bioconjugation in our other articles.
If this is the case, your best option is to either start performing some research of your own to assess whether your desired technique is possible or look for an alternative solution such as using a different amino acid or different chemical reaction.
Problems with Antibody Bioconjugation Techniques
Antibody bioconjugates have seen a significant amount of research in the field of bioconjugation science. This is because of their fascinating potential in biological imaging and immunohistochemistry. However, because of their fragile and complicated nature, there are often problems with creating bioconjugates with antibodies.
Problems with antibody bioconjugation techniques include degradation of the antibody due to harsh reagents and instability of the bioconjugate product.
Degradation Of The Antibody Due To Harsh Reagents
When trying to produce stable chemical bonds that will form the link between an antibody and another molecule, harsh chemicals can often cause the antibody to degrade. This results in an unusable bioconjugate in many cases. Here are some ways of overcoming this problem:
- Use less harsh reagents: Look for alternative reagents that you can use to create the same chemical linker between your molecule and the target antibody. This may mean something as simple as changing the solvent or more complicated solutions like finding different reagents (source).
- Experiment with the reaction conditions: You may be able to prevent the degradation of your antibody while still using the harsh reagents if you adjust your reaction conditions. Try controlling the temperature of the reaction by performing it under cooler conditions, or performing it in a different atmosphere, such as nitrogen.
Instability Of The Antibody Bioconjugate Product
If you’re successful in synthesizing your antibody bioconjugate, you might run into issues with its stability. Just like storing antibodies on their own, the conjugate may begin to decompose immediately or later on during storage. This is a difficult challenge, and the shelf-life of your conjugate product will vary depending on its specific properties. However, there are a few things you can do to extend the life of your antibody bioconjugate product:
- Cool your antibody bioconjugate: This applies to most bioconjugates. You should store your sample in cool conditions such as the refrigerator to increase its storage life. However, avoid freezing your bioconjugate, as freezing can, in some cases, damage and degrade the antibody bioconjugate compound. Experiment to see whether your sample can handle freezing.
- Use a stabilizer: There are many stabilizing compounds available that can be added to your antibody bioconjugate to extend its shelf-life. Manufacturers sell these stabilizers separately and as part of bioconjugation kits. For example, Abcam includes stabilizers in their antibody kits. You can also buy antifreeze stabilizers which allow you to store your sample in the freezer and extend its life further.
Problems with Antibody-Enzyme Conjugates
Antibody-enzyme conjugates have seen significant research in the area of immunohistochemistry and cancer-therapy. They offer unique ways to fight against cancers through precise targeting and high activity against cancer cells.
Problems with antibody-enzyme conjugates include controlling linker length and degradation of enzyme activity due to the reagents used.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Controlling Linker Length
Controlling linker length is important for antibody-enzyme conjugates because it can affect the bioconjugates stability and reactivity. Linkers can range from “zero-length” crosslinkers which are as short as effectively possible to extended length chains. If the linker is too short, it can hinder the enzyme’s activity. If the linker is too long, it may be unstable. Here are some solutions to controlling linker length:
- Experiment with different linker lengths: The most obvious solution to overcome this problem is to experiment with different linker lengths between your antibody and enzyme to assess how it affects their performance in your desired application. For example, when developing a novel protein conjugate based ELISA test for whole milk progesterone, the authors of this paper tested a range of linkers and found which was the most effective for their purpose. Learn more about bifunctional crosslinkers in conjugation in our article.
- Use a different linker: If your application requires a specific linker-length, you may need to use different bonds to improve the stability of the bioconjugate linker (source). Avoid using linkers that are easily broken in your reaction conditions. For example, avoid enzyme cleavable bonds like peptide-based linkers if you want your bioconjugate to have prolonged stability in the body.
Degradation of Enzyme Activity Due to the Reagents Used
Enzymes can be negatively affected by the reagents and conditions used in bioconjugation reactions. This can affect their enzymatic activity and potentially limit their active life. Here are some solutions to overcome this problem when synthesizing antibody-enzyme conjugates:
- Use alternative reagents: This seems obvious but it usually is the best solution. Look for an alternative reactive pathway that avoids the reagents causing the degradation of your enzyme. This may mean using alternative linkers, changing the reaction conditions (such as pH and solvent), and looking for a completely different conjugation pathway.
- Use an enzyme stabilizer: In some cases, you may be able to use an enzyme stabilizer to minimize the degradation of your enzyme due to the reagents you’re using to produce the bioconjugate. These stabilizers can protect against reactive and thermal stress. For example, major suppliers such as Sigma and Thermo supply these types of stabilizers. If these types of stabilizers don’t solve your problem, consider delving into the available literature on enzyme stabilization, such as in this paper, to learn more about your options.
Bioconjugation Problems with Protein-DNA Conjugation Techniques
DNA and protein structures are two of the most important classes of biomacromolecules in biology. The bioconjugation of these structures opens up many new options which combine the proteins of both DNA and proteins. This gives access to tools such as molecular recognition, enzymatic catalysis, and structural hybridization. However, there are numerous challenges you might face when creating protein-DNA bioconjugates. We discuss DNA bioconjugation techniques in more detail in another article.
Bioconjugation problems with protein-DNA conjugation techniques include the introduction of reaction sites to the DNA and low reaction yields.
Introduction of Reaction Sites On DNA
The conjugation of DNA to a protein isn’t always straightforward. It often requires the introduction of reaction sites to the DNA structure which can be challenging depending on your applications. Here are some solutions to the problem of needing to introduce reaction sites on DNA:
- Direct chemical modification: The simplest way to add reaction sites to DNA is to perform a direct chemical reaction to add the desired functional groups to its structure. However, this can often be non-specific or have unintended effects on the DNA structure (source).
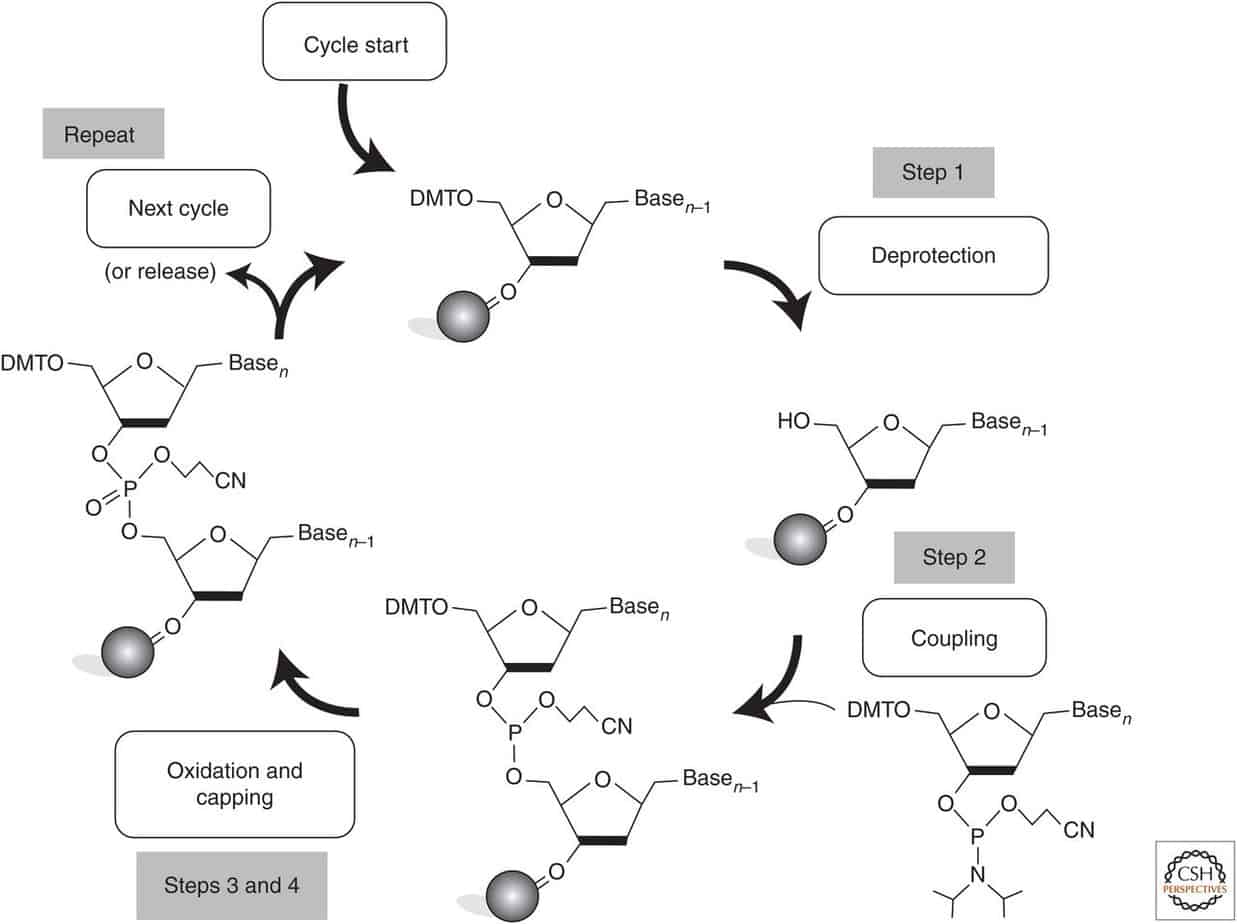
- Synthesize DNA with your desired functionality: If you have the facilities to synthesize DNA, you can add the desired functionality to the structure by modifying the DNA chain during its synthesis (source). If you want to learn more about click-chemistry, read our article on orthogonal bioconjugation techniques.
Low Reaction Yields
When synthesizing protein-DNA conjugates, one of the most frustrating problems you can face is low reaction yields. The reaction is working but it is only producing a small amount of your desired bioconjugate compound. Here are some ways to overcome this:
- Optimize your reaction conditions: The first and most important thing to do when facing low reaction yields from a protein-DNA conjugation reaction is to begin actively experimenting with your reaction conditions to see if you can improve the yield. Start with simple tweaks such as the reaction time, temperature, and solvent. If this problem persists, consider searching the literature for similar reactions and making adjustments based on what you can find.
- Try an alternative purification technique: If your yields are low after purification, you may be losing your bioconjugate during the purification step. This can often be a problem if there are unreacted proteins and DNA in the mixture. Look for alternative purification techniques, like this one.
