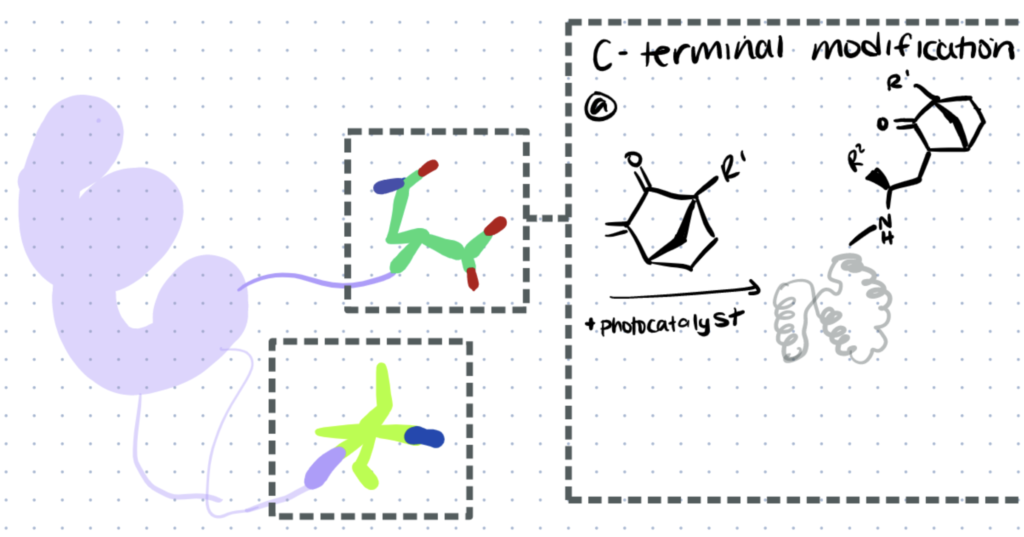
Bioconjugation that occurs at the C- terminus site of proteins and peptides is known as C- Terminal bioconjugation. In this article we’ll discuss different types of C-terminal bioconjugation protocols.
Methods for C-Terminal bioconjugation of proteins and peptides include photoredox catalyzed decarboxylative alkynylation, thioacid/azide amidation and chemoenzymatic strategies that utilize Asparaginyl Endopeptidase.
What Is The C-Terminus?
The C-terminus (also known as the carboxyl-terminus, C-terminal end, carboxy-terminal tail, or COOH-terminus) is the end of an amino acid chain (polypeptide or protein).
Minimotifs, small peptides with an encoded function such as binding, posttranslational modifications, and trafficking, are found at the C-termini of proteins. Many of these activities are unique to C-terminal minimotifs.
C-Terminal Bioconjugation Protocols
There are numerous methods for modifying the C-terminus of a protein.
For example, one method for C-terminal bioconjugation is to use native chemical ligation, which has high yields and is a flexible technique. In this technique, the first step involves interaction between a thiol and a thioester and is reversible. The second step involves formation of an amide and is irreversible. Using NCL, it’s possible for a C-terminus on one protein to be linked to a thiol group on a cysteine residue on another protein’s N-terminal. You can use this technique to create large polypeptides by conjugating together multiple smaller ones. The final ligation product yields are quite high (source).
There are also several electrochemical bioconjugation methods and enzymatic methods (such as transglutaminase bioconjugation) that are site-selective for the C-terminus.
We’ll talk about several other methods below along with providing references and high level steps. We have also linked to research articles where you can find more detailed information.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
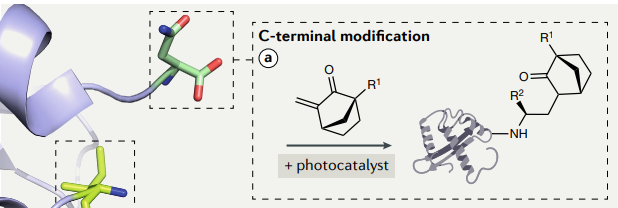
1. C-Terminal Bioconjugation Of Peptides Through Photoredox Catalyzed Decarboxylative Alkynylation
Photoredox-catalyzed decarboxylation of amino acids can be achieved using ethynylbenziodoxolone (EBX) reagents (source). These reagents have also been used in alkynylation of thiols in cysteines and in alkynylation of indoles in tryptophan.
Below is a representation of the process of decarboxylative alkynylation of peptides using EBX reagents and specific organic dyes which are used as photocatalysts. The reaction is clean, quick, free of metal, and produces alkynylated peptides with a wide tolerance for C-terminal amino acids. Using this method, an R-group can be installed at the C-terminus and used to react with any other molecule.
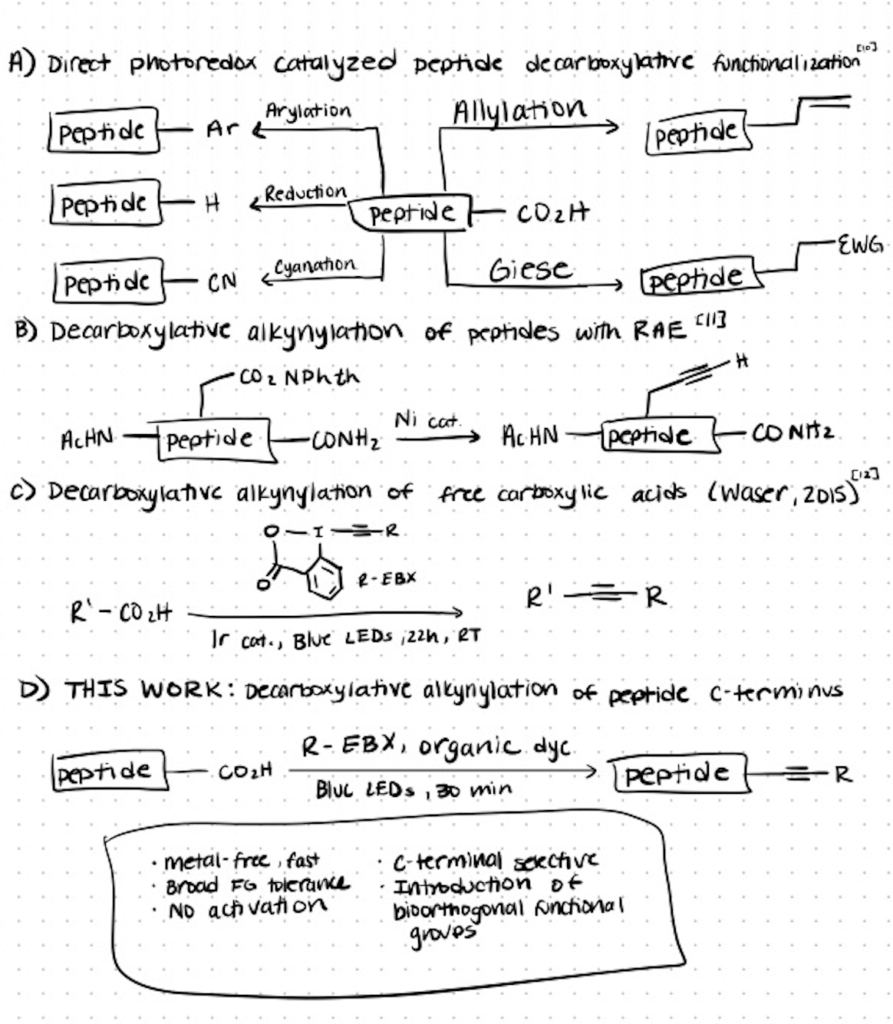
The steps of C-Terminal bioconjugation of peptides through photoredox catalyzed decarboxylative alkynylation are as follows. You can find the detailed process in the original article (source):
Step 1. Preparation Of Hypervalent Iodine Reagents
NaIO4 (40.5 g, 189 mmol, 1.05 equiv) and 2-iodobenzoic acid (14) (44.8 g, 180 mmol, 1.0 equiv) are suspended in 30% (v:v) aq. AcOH (350 mL) and combined, diluted, and filtered, after which the crude product is collected.
Step 2.Preparation of Organic Dyes
A 60% suspension of sodium hydride in mineral oil (8.0 equiv) is slowly added to a stirred solution of substituted-carbazole 35a-d (5.0 equiv) in dry THF (0.05 M) under a nitrogen atmosphere at room temperature. Added to this is 2,4,5,6-tetrachloroisophthalonitrile 36 (1.0 mmol, 1.0 equiv). The mixture is concentrated, recrystallized, and purified.
Step 3. Synthesis Of Peptide Tetramers
Using a 2-chlorotrityl chloride resin (1.0-1.6 mmol/g, 100-200 mesh), peptide tetramers are synthesized by solid phase peptide synthesis. An initial amino acid is introduced onto the resin by incubation of the Fmoc-protected monomer (3 equiv of the number of active sites on the resin), DIPEA (4 equiv) in dichloromethane. Precipitated peptides are collected, refined, and purified.
Step 4.Decarboxylative Alkynylation
Degassed solvent (10 mL) is then used to suspend the peptides (1.0 equiv), and Ph-EBX (1.5 equiv), the base and the catalyst, under a nitrogen atmosphere. The resulting mixture is then irradiated.
2. Thioacid/Azide Amidation
Techniques for site-selective protein modification at the C-terminus are key tools for studying protein structure-function and drug discovery. Such approaches, for example, can be employed to mark proteins with a biophysical probe for structural and biochemical studies or with a biocompatible polymer for the creation of protein-based medicinal treatments. They can also be used to immobilise proteins and tie them to solid supports in the manufacture of protein chips. Below we discuss a method written by Zhang et al at a high level. For more details, refer to the original paper.
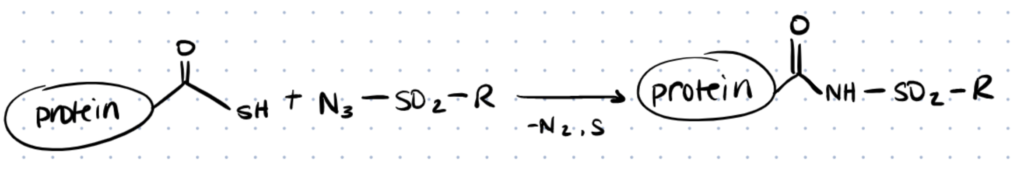
Step 1. Choose a Protein to Modify at the C-Terminus
In this article, the researchers chose the tiny ubiquitin protein. Obtaining a sufficient amount of thioacid protein is a critical first step. Sometimes you may need to create a protein with a C-terminus thioacid. For this, splice in a cysteine into the protein structure using saturation mutagenesis.
Step 2. Clone And Purify The Protein Using A Fusion Protein Method
Overexpression of the ubiquitin-intein-CBD fusion protein in E. coli was followed by affinity purification using chitin beads. The bound fusion protein was incubated in the hydrothiolytic cleavage buffer (0.1 M Na2S, 0.25 M HEPES, 0.5 M NaCl, 1 mM EDTA, pH 8.0) overnight at 37 °C to cleave ubiquitin from the fusion protein. These were the same conditions employed in the hydrothiolysis of synthesised peptide thioesters.
Step 3. Cleave The Disulfide Linker Of The Fusion Protein
Begley et al. prepared a 66-residue protein thioacid ThiS-COSH by cleaving it with 30 mM ammonium sulphide at 4 °C in earlier work. The cleaved ubiquitin thioacid was isolated using RP-HPLC after being eluted from chitin beads. The existence of the thioacid group was confirmed by high-resolution MS analysis. The ubiquitin thioacid yield was usually around 4-5 mg per litre of cell culture.
Step 4. C-Terminal Bioconjugation of PEG
For putting a PEG moiety onto the C-terminus of the recombinant ubiquitin, benzenesulfonazide-functionalized tagging agent was produced to see if thioacid amidation with big sulfonyl azides would work. In an aqueous solution, the amidation reaction of ubiquitin thioacid with PEGylation reagent was carried out: 0.3 mM ubiquitin thioacid, 2 mM PEG sulfonazide 1 in 6 M Gdn-HCl containing 3 mM 2,6-lutidine. At room temperature, the reaction took around 10 hours to complete and was followed by some hydrolysis. This is a common issue with water-based reactions. Learn more about challenges with bioconjugation in water, here.
Step 5. Use HPLC Analysis to Assess Bioconjugation at the C Terminus
When the reaction was carried out in a HEPES buffered solution (pH 7 or 8), there was greater hydrolysis and the hydrolyzed ubiquitin-COOH appeared soon after the ubiquitin thioacid was combined with the azide.
As a result, the authors used 2,6-lutidine to reduce the amount of ubiquitin thioacid hydrolysis. According to HPLC analysis, the PEGylation reaction yield was around 65 percent. Despite using an excessive amount of PEGylation agent, the reaction produced just the mono-PEGylation product, which was validated by MS analysis, demonstrating that tagging PEG to the protein was extremely chemoselective and site-specific.
3. C-Terminal Bioconjugation of Peptides Through Asparaginyl Endopeptides
The catalytic efficacy of asparaginyl endopeptides (AEP) makes them attractive tools for protein bioconjugation.
In this article, the authors utilize AEP along with a Asn-Cys-Leu recognition sequence to make it easy to modify the C-terminus and N-terminus of 5 different protein samples.
Step 1. Express OaAEP1
First, the authors express OaAEP1 in E. coli strain BL21.
Step 2. Use Nickel Columns to Purify the Enzyme
To purify Ni-NTA elution fractions containing the protein, ion-exchange chromatography with 25 ml of Q-sepharose was utilised (GE Healthcare). Over four column volumes, a continuous salt gradient of 0–50 percent of buffer B: 20 mM Bis-Tris propane (pH 7.0), 2 M NaCl was utilised to elute bound proteins.
Step 3. OaAEP1 Activation
The concentrated protein stock was diluted to 1.5 M with 50 mM sodium acetate (pH 4.0), 50 mM NaCl, 1 mM EDTA, and 0.5 mM TCEP-HCl buffer. The activation mixture was then dialyzed at 20 degrees Celsius (sodium acetate buffer as described above).
Step 4. Bioconjugation to The C-Terminus
You can follow which sequences of proteins the researchers modified with the Asn-Cys-Leu recognition sequence in the supplementary section here. Then they simply incubated the enzyme with the different substrates (peptide sequences and proteins) and were able to selectively attach to the C-terminus.
Applications Of C-Terminal Bioconjugation
Applications of C-Terminal bioconjugation include the attachment of therapeutic cargo to proteins, bioconjugation of drugs to create antibody-drug conjugates, and the creation of imaging probes.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Application 1. Attach Therapeutic Cargo To Proteins
Chemical modification of proteins is a field of chemical biology that is fast growing.
Biochemical probes installed selectively onto the C-terminus have improved our understanding of endogenous protein modification and macromolecular function. In certain situations, chemical modification can even completely alter the function of a protein.
For example, you can hijack a normal bacterial protein and attach a therapeutic cargo to it to fight HIV, cancer, malaria, and bacterial infections (source).
Application 2. C Terminus Bioconjugation for Antibody Drug Conjugate
Antibody–drug conjugates (ADCs) are targeted therapeutics that combine the specificity of antibodies with the potency of small molecules.
The Neri group has modified cysteine residues at the C-terminus of each CH4 domain of small immunological proteins. Using an umpolung technique, the cysteine residues are conjugated in a selective and traceless manner.
Selective reduction is then performed with TCEP, and the C-terminal cysteine residues are oxidised with Ellman’s reagent. After treating the electrophilic disulfides with thiol-functionalized cematodin, site-specific modified proteins are obtained with high conversions and fast reaction times.
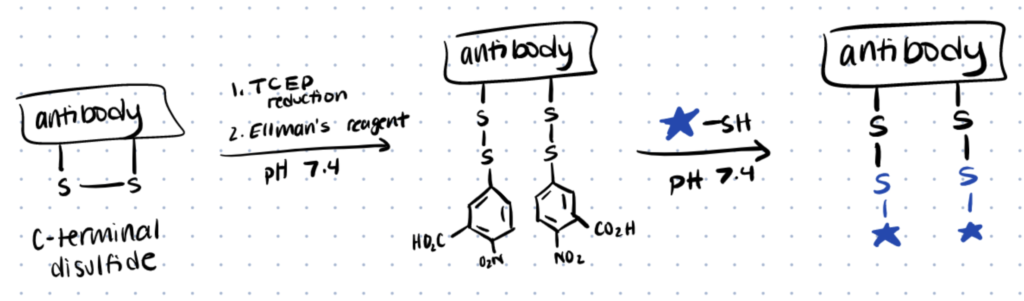
You can reduce C-terminal disulfides using TCEP and then react with them. However, you need to engineer the C-terminal disulfide into the antibody. Source: ResearchGate. ALT: C-terminal bioconjugation to antibodies
Application 3. C-Terminal Bioconjugation Use in Diagnostic Imaging
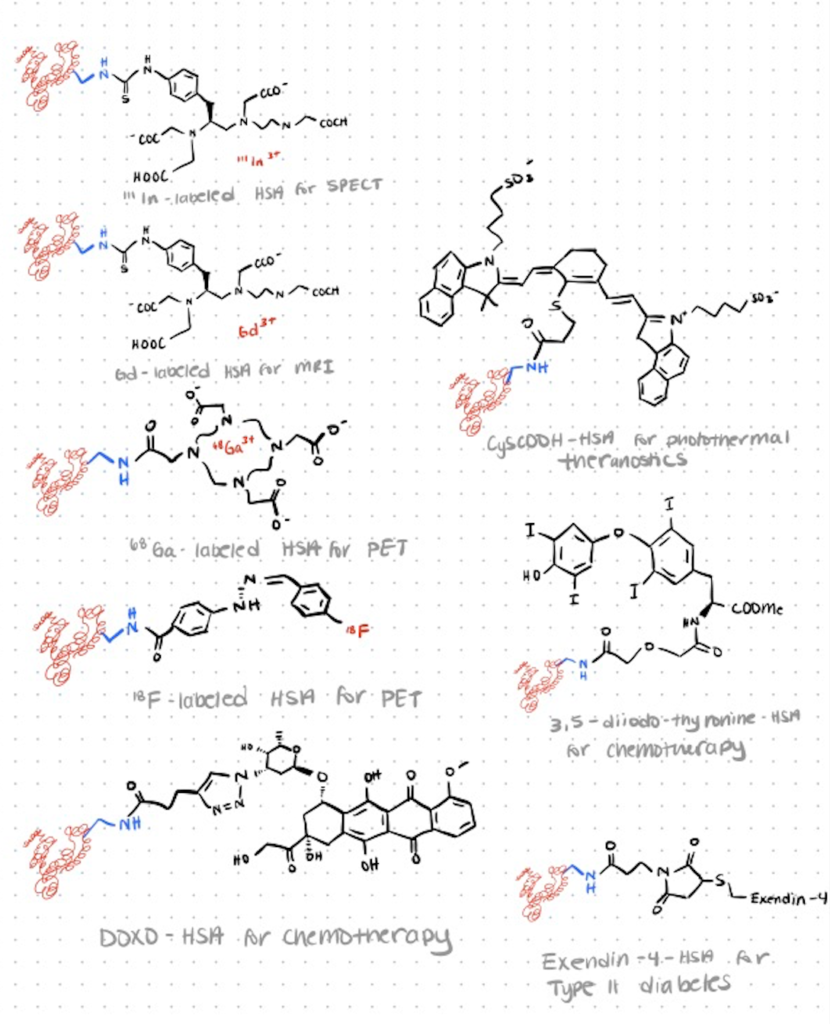
HSA (Human serum albumin) is the most abundant protein in plasma and can be used as a diverse drug delivery vehicle as well as a way to extend the active profile of fast-clearance drugs. It transports several vital macromolecules, including fatty acids, hormones, and amino acids, in addition to being a major drug delivery protein in blood.
In blood circulation, HSA has a rather lengthy half-life (19 days). Small molecule medications and imaging probes are commonly carried by HSA because it is biodegradable, non-toxic, and non-immunogenic, making it a good candidate for use as an excipient in vaccines and other medications.
HSA-based diagnostic agents are commonly prepared using in vitro covalent conjugation. You can radiolabel HSA with radiometals for diagnostic imaging by using NHS-activated ester of DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and incubating with HSA under mildly basic conditions (pH = 8–9). You can also attach HSA to quantum dots; we’ve discussed bioconjugation of quantum dots in detail in another article.
Using positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI), F-HSA, Ga-DOTA–HSA, In-DTPA–HSA and Gd-DTPA–HSA have all been imaged (source). Note that DTPA is the abbreviation for diethylene triamine pentaacetic acid.
Other Methods For Reacting With C-Terminal Amino Acids
In some cases you can utilize specific bioconjugation strategies if you know which amino acid is at the C-terminus. For example, you might consider tyrosine bioconjugation methods if there’s a C-terminal tyrosine residue, or arginine bioconjugation if there’s an arginine there.
Peptide Conjugation to Carrier Proteins Using EDC/NHS
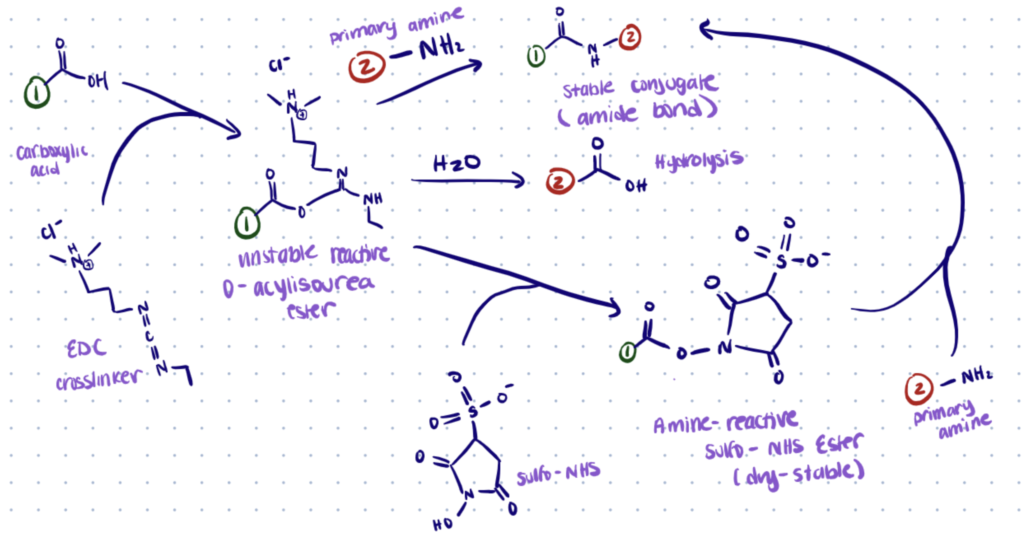
You can use a classic chemistry like EDC/NHS to react with C-terminal amino acids. However, it’s a pretty poor chemistry which has low yields and might denature your protein of interest if it’s not stable at low pH.
EDC forms an active O-acylisourea intermediate when it combines with carboxylic acid groups like those at the C-terminus of proteins. Once the reaction has occurred, the intermediate is easily displaced by nucleophilic attack from primary amino groups in the reaction mixture. An EDC by-product is produced as a soluble urea derivative when an amide bond is formed between the primary amine and the original carboxyl group.
In aqueous solutions, the O-acylisourea intermediate is unstable. Failure to react with an amine results in hydrolysis of the intermediate, carboxyl regeneration, and the release of an N-unsubstituted urea. EDC crosslinking works best in an acidic (pH 4.5) environment and when using buffers free of extraneous carboxyls and amines. A suitable carbodiimide reaction buffer is MES buffer (4-morpholinoethanesulfonic acid). Phosphate buffers can be used in neutral pH (up to 7.2) conditions in reaction chemistry, but with less efficiency. Increasing the amount of EDC in the reaction solution can compensate for this decreased efficiency.
To boost efficiency or develop dry-stable (amine-reactive) intermediates, EDC coupling procedures frequently contain N-hydroxysuccinimide (NHS) or its water-soluble equivalent (Sulfo-NHS). NHS is conjugated to carboxyls by EDC, resulting in an NHS ester that is much more stable than the O-acylisourea intermediate, and allows for effective conjugation to primary amines at physiological pH.
C-Terminus Bioconjugation By Visible-Light-Mediated Single-Electron Transfer (SET)
The differential in oxidation potential between terminal carboxylic acids and in-chain Glu and Asp residues is used for C-terminal bioconjugation. MacMillan and colleagues have described a method for performing decarboxylative alkylation on C-terminal residues using visible-light-mediated single-electron transfer (SET) (3 equiv. photocatalyst, 10 equiv. Michael acceptor, for 8 hours, at room temperature, with a pH 3.5, and a 41–49 percent conversion).
Unlike previous amide coupling and esterification techniques, the SET reaction favors the C-terminus of Glu and Asp residues due to the higher stability of the C-terminal carbon-centered radical. Human insulin can be selectively alkylated at the C-terminus using the SET reaction.