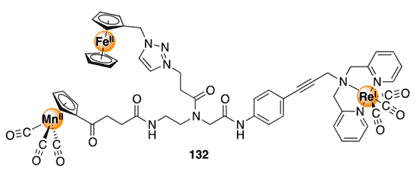
An immense number of extensively complex and interconnected biological processes are governed by metal ions. One of the principal themes of bioinorganic chemistry is the synthesis of metal complexes that have the ability to mimic the functional properties of natural metallic systems. Under these circumstances, a variety of metal complexes have been attached to various biomolecules to increase their compatibility/selectivity with biological systems. In this article we’ll discuss protocols for bioconjugation of metal complexes so you can start utilizing metal complexes in your research.
Protocols for bioconjugation of metal complexes include chemoenzymatic methods using Sortase A, photochemical methods using aryl-azides, and the electropolymerization methods.
In this article, we’ll describe some recent synthetic strategies by which metal complexes can be conjugated to biomolecules via highly selective and bioorthogonal transformations, and we’ll provide some common applications of such conjugates. These methods provide new possibilities for selective reactivity to escape from the nucleophile–electrophile strategies such as hydrazine bioconjugation and cycloaddition manifolds such as azide bioconjugation and click chemistry that dominate traditional bioconjugation reactions.
What are Metal Complexes?
Metal complexes consist of a central metal ion that is bonded together with a number of atoms, ions, or neutral molecules called ligands via “coordinate-covalent bonds.”
The number of ligands bound to the transition metal ion is called the coordination number, and the naming system incorporates prefixes to indicate the number of ligands of each type coordinated to the metal (Ndagi et al., 2016; Nandy et al., 2021; Garnovskii et al., 2002).
Metal complexes possess the ability to coordinate ligands in a three-dimensional configuration. The number and the large structural variety of these complexes allow the functionalization of groups that can be shaped to defined molecular targets.
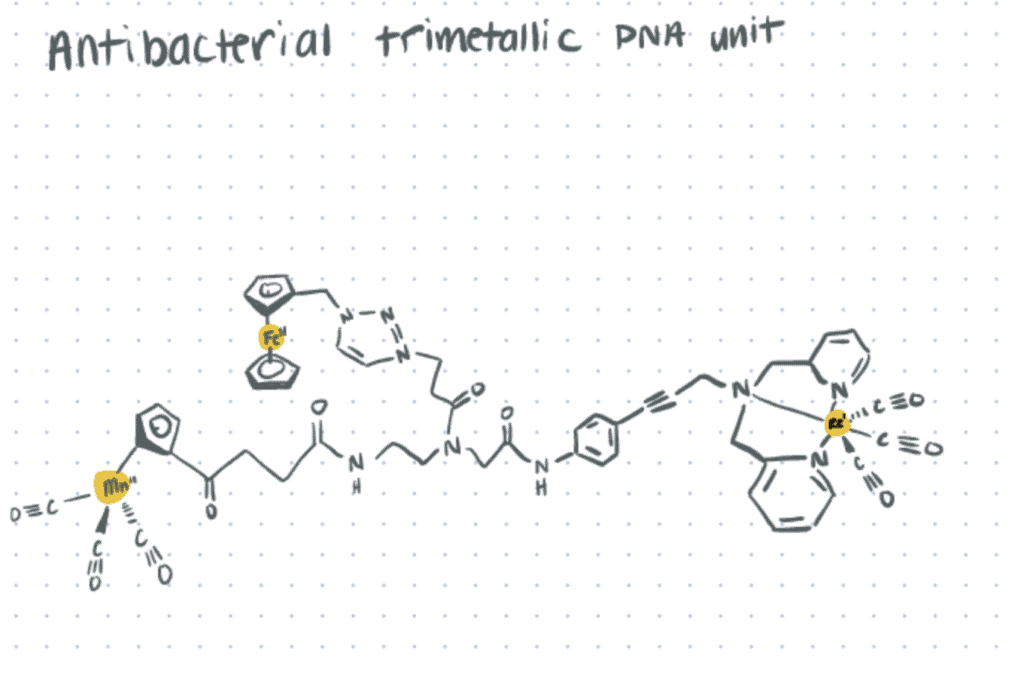
Organometallic Complexes
Organometallic complexes differ from traditional metal complexes by containing at least one covalent bond between a metallic element and a carbon atom belonging to an organic molecule. Organometallic complexes can act both as a nucleophile and a base as the electronegativity of the metal is very low compared to carbon–highlighting the importance of the electropositive character of the metal. The metal involved in the formation of the organometallic chemical bond can be an alkali metal, alkaline earth metal, transition metal, or a metalloid.
Organometallic complexes are widely employed as stoichiometric reagents and homogeneous catalysts for various industrial chemical reactions (Mudi et al., 2015). Nowadays, a large number of organometallic conjugated polymer networks have also been synthesized and used for the production of semiconductors, LED lights, solar cells, and various therapeutic products.
Protocols for Bioconjugation of Metal Complexes
Some protocols for bioconjugation of metal complexes include enzyme-mediated site-specific bioconjugation using Sortase A, photochemically-induced bioconjugation using photoactive substrates, and electropolymerization.
Method 1. Enzyme-Mediated Site-Specific Bioconjugation Of Metal Complexes
Radioactively labeled mAbs and short-chain antibody fragments (scFv) combine the diagnostic and therapeutic possibilities of nuclear medicine with the exquisite selectivity of antibody targeting. Given the complex nature of antibody conjugation, nonspecific binding is particularly problematic.
In contrast to the conventional antibody conjugation methods of lysine/cysteine residues on the antibody surfaces, chemoenzymatic modifications of antibodies have been shown to offer highly site-specific, robust, and reproducible conjugation reactions under mild conditions to ensure the maintenance of the biological activity of the antibodies.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
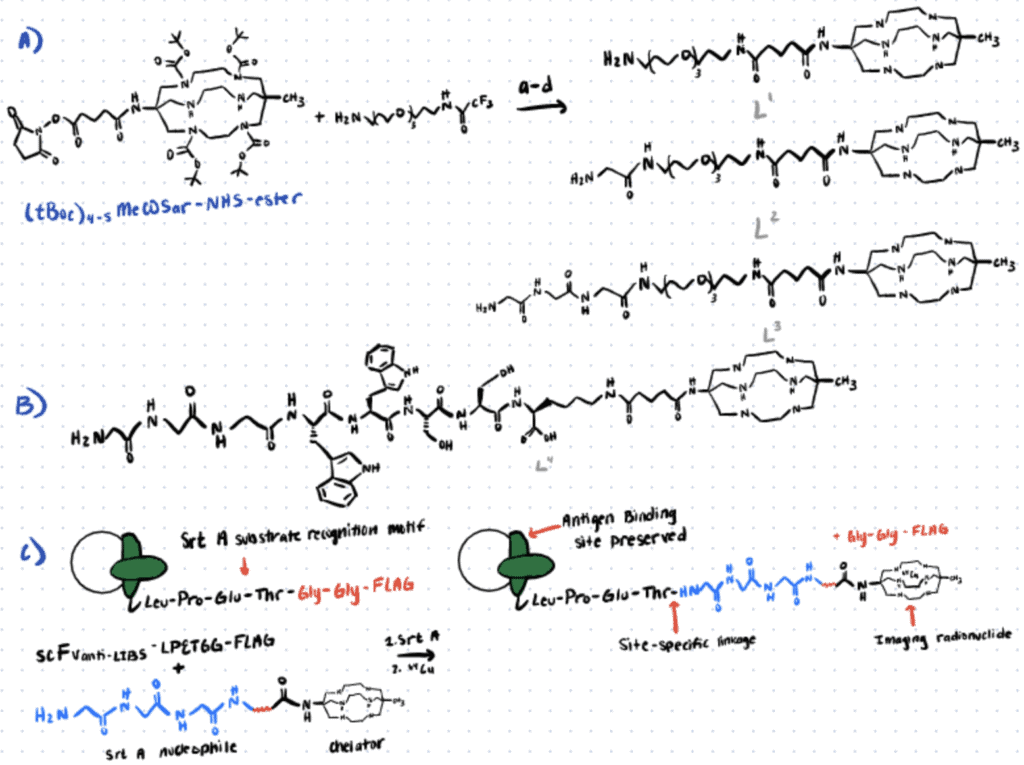
In the following section, we briefly describe a sortase-mediated 64Cu-antibody bioconjugation method utilized by Paterson et al. (2014). For more details, refer to this article.
Step 1. Synthesis Of Enzyme Substrates
In this study, glycine-containing bifunctional chelator MeCOSar derivatives were synthesized via NHS-ester mediated reaction and used as the substrates for sortase A.
Step 2. Sortase A-Mediated Bioconjugation
The bifunctional chelator was allowed to react with the antibody fragment scFvanti-LIBS in the presence of the sortase A for five hours at 37oC. The purified product was generated in good yield and the flow cytometry analysis of the immunoconjugates also indicated strong binding to the target.
Step 3. Radiolabeling Of Immunoconjugates
The immunoconjugates were then radiolabeled with 64Cu2+ (pH 7, RCP >95%, room temperature) for less than 30 minutes. In vivo, PET-imaging and post-mortem biodistribution results confirmed that the targeting abilities of the radiolabeled immunoconjugates are highly specific.
Method 2. Photochemically-Induced Bioconjugation of Metal Complexes
Recently, bioconjugation methods using visible-light photocatalysis of a photoactive substrate have emerged as powerful synthetic tools for the rapid and selective functionalization of biomolecules under mild reaction conditions. As most site-specific bioconjugation reactions available nowadays mainly rely on thermochemically-mediated multiple-step conjugation processes, and given the time constraints of working with radionuclides, photochemistry has proven indispensable in the synthesis of radiolabeled antibodies, immunoglobulin fragments and other proteins/peptides (Holland et al., 2020; Haas & Franz, 2009; Ihara & Kitamura, 2012).
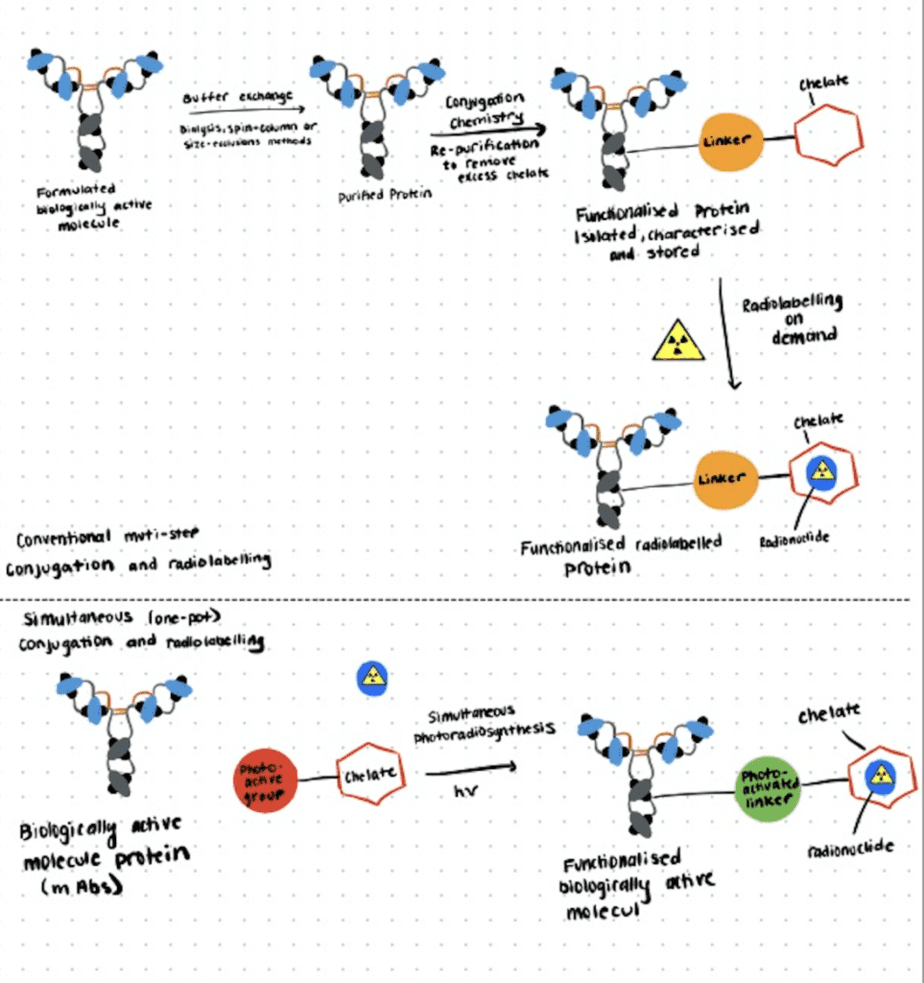
In the following section, we briefly describe a photo radiochemical labeling approach for antibodies introduced by Patra et al. (2019). For more details, refer to this paper.
Step 1. Chemical And Radiochemical Synthesis
In this study, a photoactivatable chelate based on the functionalization of desferrioxamine B with a photoreactive aryl azide moiety (DFO-ArN3) and the radiolabeled complex (89Zr-1+) were synthesized and characterized using standard methods
Step 2. One-Step Photochemical Bioconjugation
To induce the radiolabeling and photochemical conjugation reactions simultaneously, the authors directly mixed the radioactive compound, the chelator, and the antibody trastuzumab in water (pH 8-9), followed by irradiation with LED source (365 nm, pH 8-9, room temperature). The photochemical degradation kinetics of the chelator were consistent with the established mechanism of aryl azide photoactivation as described by Gritsan and Platz (2006).
Step 3. Characterization Of Radiolabeled Immunoconjugates
In contrast to the two-step approach, the one-step process gave a significantly higher photoradiolabeling efficiency while also providing the unique advantage of avoiding the need to isolate, store, and characterize the conjugated intermediate antibodies. The following in vivo and ex vivo PET-imaging and biodistribution studies also demonstrated that the photo radiolabeling methods are viable for the synthesis of radiolabeled antibodies.
Method 3. Electropolymerization of Metal Complexes
Electropolymerization represents a straightforward and well-established approach for the assembly of polymer structures with enhanced stability, high surface area, and controllable thickness. This method offers several advantages compared to common polymerization techniques (Friebe et al., 2012). Nowadays, a broad range of both electropolymerizable monomers and metal complexes with various structures are available commercially.
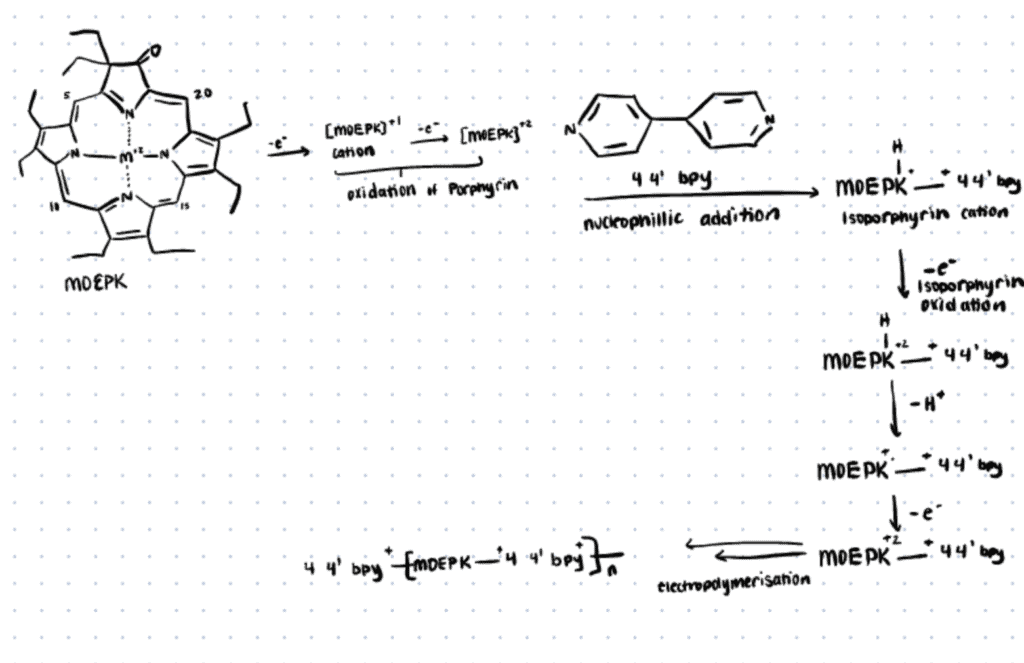
In the following section, we briefly describe an electropolymerization method utilized by Kochrekar et. al. (2021) For more details, refer to this link.
Step 1. Materials Preparation
β-keto functionalized metal porphyrins used in this study, namely the zinc octaethyl porphyrin ketone (ZnOEPK) and nickel octaethyl porphyrin ketone (NiOEPK) were synthesized according to the previous report (Papkovsky et al., 1996; Chang & Sotiriou, 1985). The molecular catalyst 4,4′-bipyridine was used as the bridging nucleophile that influences the overall electronic structure and activity of the system.
Step 2. Electrochemical Measurements
Cyclic voltammetry was performed based on the conventional three electrodes system. One-sided fluorinated indium tin oxide (sheet resistance 8.1 Ω sq−1 ) and coiled platinum wire were used as the working and counter electrodes, respectively.
Step 3. Electropolymerization
All the polymer films were formed by 25 iterative potentiodynamic cycles in porphyrin (NiOEPK and ZnOEPK) and 4,4′-bpy solution (−0.6-1.6 V, 200 mV s−1).
Step 4. Polymer Characterization
Electropolymerization of porphyrin in the presence of an appropriate bridging nucleophile occurred via nucleophilic attack of the bridging nucleophile at the active meso position leading to the formation of polymers (Giraudeau et al., 2009; Schaming et al., 2011). The polymer films were characterized by electrochemical, spectroscopic, and imaging techniques.
Applications of Metal Complexes
Metal complex bioconjugates are used in applications such as metal-based chemotherapeutic agents, bioanalytical probes, and as complex dyes for industrial processes and food production.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Application 1. Bioconjugation among Metallopharmaceuticals
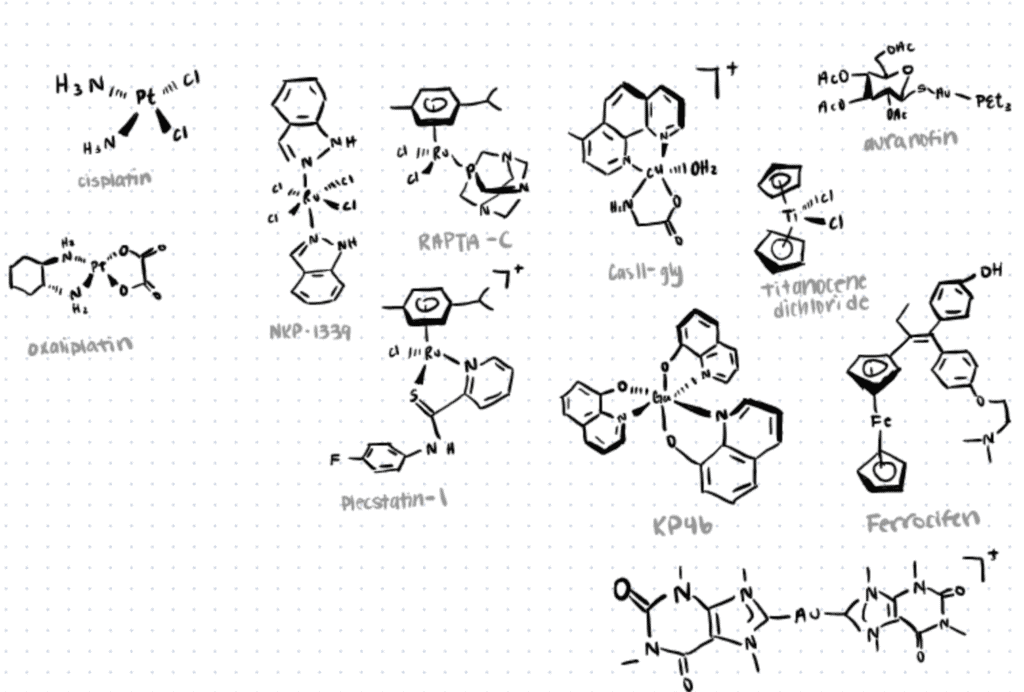
Research has shown significant progress in the utilization of novel metal-based chemotherapeutic agents that offer unique therapeutic opportunities in treating diseases such as cancer, tumors, diabetes, and many more (Jurca et al., 2017: Sodhi & Paul, 2019; Meier-Menches and Casini, 2020). One of the most popular examples in this field is the design and preparation of gold-based drugs, which have gathered increasing attention due to their strong inhibitory growth effect in tumor cells. Ag(I) complexes have also received special attention for their antitumor activity and low toxicity exhibited by their complexes.
Application 2. Metal Complex Probes
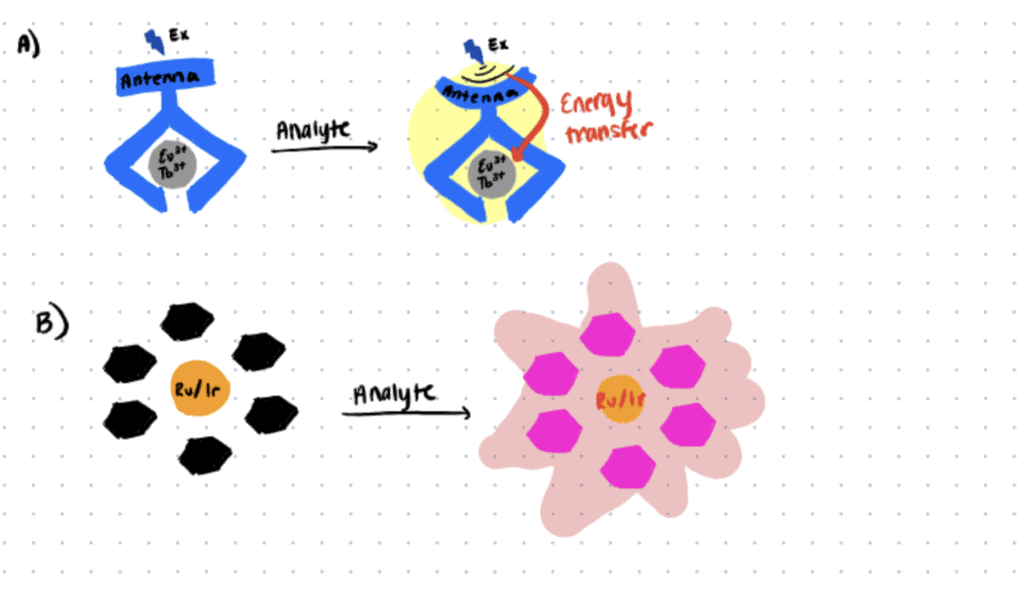
The development of reliable bioanalytical probes for selective and sensitive bioimaging and biosensing is essential for a better understanding of the dynamics of biomolecules in native systems. Taking advantage of their unique spectral/temporal properties and controllable permeability, metal complex probes have served as the basis of various bioimaging techniques for decades (Zhang & Yuan, 2020; Reyes et al., 2021; Ning et al., 2022) .
One of the most recent advances in the development of metal complex probes is the lanthanide (Eu(III)/Tb(III)) and transition metal (Ru(II)/Ir(III)) complexes for the development of responsive TGL probes. These reversible next-generation TGL probes have been shown to exhibit higher biocompatibility, and the ability to be excited using lower-energy photons that allow for long-term tracking of the biomolecules in situ in deep tissue with minimal perturbation to the native biological microenvironment (Zhang & Yuan, 2020).
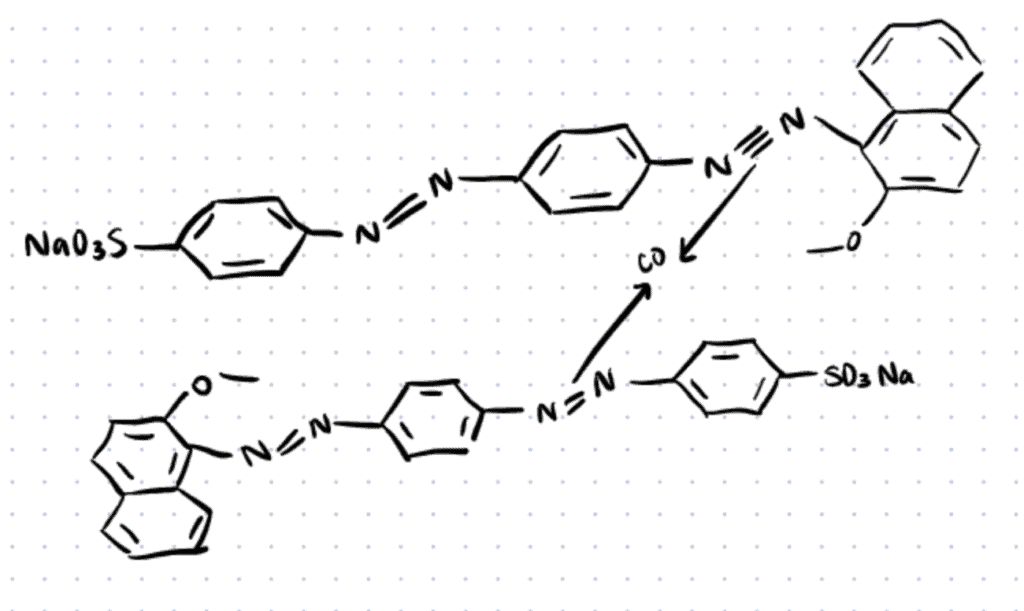
Application 3. Metal Complex Dyes
Many azo dyes possess selective metal complex chelating sites that allow them to form strong coordination complexes with bi-/polyvalent transition metal ions such as copper, cobalt, nickel and chromium. As colorant materials, metal complex dyes are widely used for industrial, printing, food, and cosmetic industries due to their show enhanced photophysical/colorant properties and greater affinity towards fibers relative to their parent dyes (Clark, 2011; Chakraborty, 2011; Dharmalingam et al., 2011). One of the most recent innovations in metal-based azo dyes, is the new Co2+ complex using the acid red 151 that expressed excellent fastness to rubbing, washing, perspiration, and light of dyed fibers when applied to nylon fibers (Ali & El-Megied, 2021).