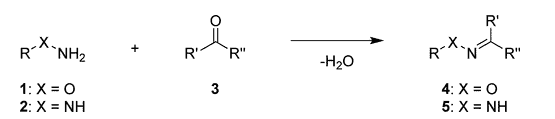
Hydrazine bioconjugation is an extremely useful method of bioconjugation used for applications in pharmaceuticals, nanomaterials and many other disciplines. The formations of oximes and hydrazones are commonly used for their simplicity and mild linking reactions.
Hydrazine bioconjugation involves the linking of hydrazine to a ketone or aldehyde which can then be reversed by hydrolysis by changing the pH. Hydrazine conjugates can be used for many applications including anticancer therapeutics, anti-inflammatory drugs and antiviral systems.
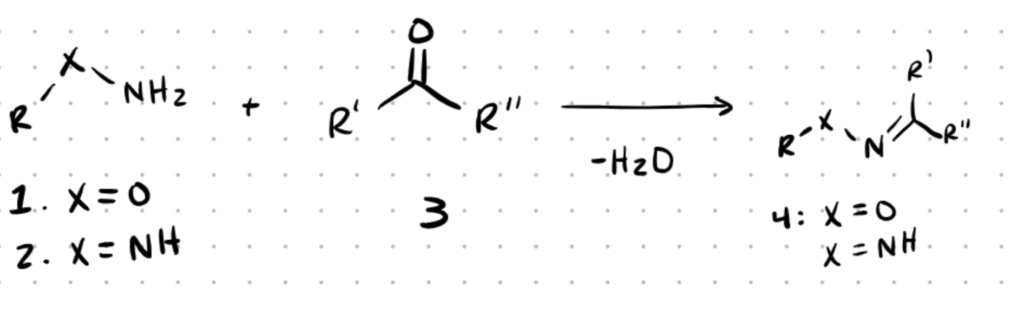
Hydrazine Bioconjugation Chemistry
To undergo hydrazine bioconjugation, a molecule must have a carbonyl moiety or an alpha nucleophile present. Biomolecules that qualify will undergo a simple reaction under mildly acidic conditions to form hydrazones.
Typically, hydrazine bioconjugation chemistry involves reaction of the NH group of the amine with aldehydes and ketones on biomolecules to form an imine.
Another interesting amine reaction, ring-opening, is discussed in our article, bioconjugation using epoxides.
Hydrazone Formation with Aldehydes and Ketones
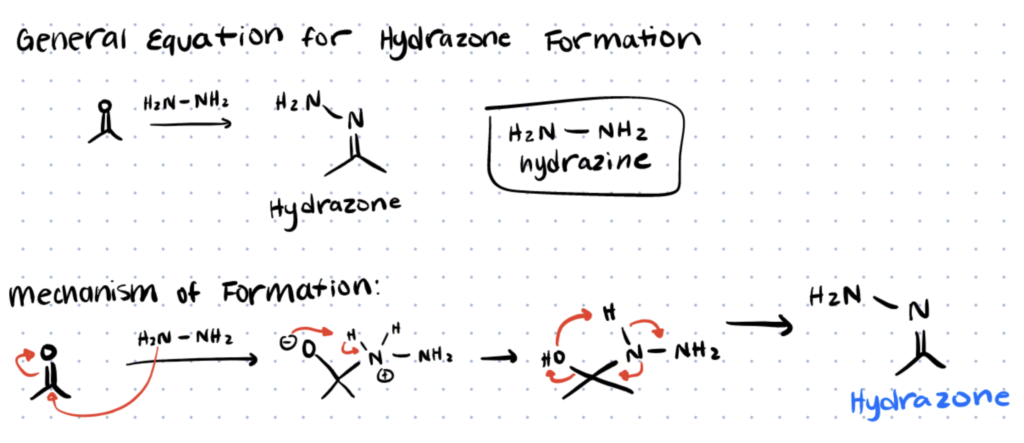
The figure below shows the reaction mechanism for the reaction of hydrazine with an aldehyde or ketone to form hydrazone. The reaction starts with a nucleophilic attack by hydrazine which breaks the double bond of the carbonyl group, resulting in a positive charge on the nitrogen and negative charge on the oxygen. The electron delocalization allows for hydrogen atoms to be donated to the oxygen group, resulting in formation of a water and formation of an imine to form the final hydrazone complex.
Hydrazines have better reaction kinetics with aldehydes than ketones, making aldehydes ideal for hydrazone formation. Additionally aryl aldehydes tend to perform better than aliphatic aldehydes having faster kinetics and better stability. These reactions happen quickly under slightly acidic pH, however a pH less than 3 will slow reaction kinetics hindering the extent and rate of reaction. Additionally reactions occurring at a pH greater than or equal to 7 require a catalyst to drive the reaction but will be more selective and produce stable products.
The Difference between Hydrazine vs. Hydrazone
Although it is easy to confuse hydrazine with a hydrazone the key difference is that a hydrazine is an amine that is used to form a hydrazone. A hydrazone refers to a molecule that has been formed by reacting a hydrazine with a ketone to form the hydrazone bond.
Hydrazone Hydrolysis Mechanism
The resulting hydrazone is very stable at alkaline pH, however by adjusting the pH to acidic conditions and having water in excess it will quickly hydrolyze and result in cleavage of the imide group. This mechanism is used for pH controlled drug delivery systems. The hydrolysis reaction happens in the exact opposite manner starting with protonation of the imide nitrogen and then nucleophilic substitution by a water. The ease at which this reaction will occur can be controlled by pH, electronic, and steric hindrance. Hydrazones formed from ketones tend to be more stable. Studies have shown that the stability can be further increased by producing trimethylhydrazinium ions or using oxime’s instead, offering diverse characteristics for many applications.
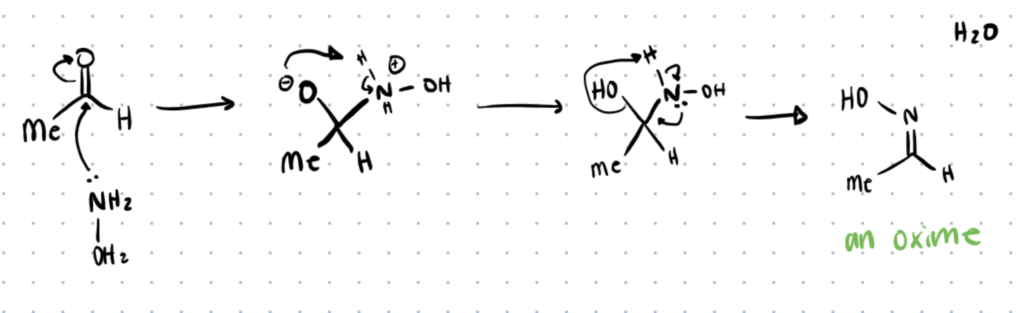
Oxime Formation Mechanism
Oxime formation is very similar to the synthesis of hydrazones. First there is a nucleophilic attack of the aldehyde or ketone by oxyamine, followed by protonation of nitrogen to form an oxime bond and water. Similar to hydrazones, aldehydes tend to react quicker than ketones, however oximes tend to be much more stable than hydrazones with rates of hydrolysis up to 1000 times slower than that of hydrazones. This increased stability can be explained by the greater nucleophilicity of oxime by the presence of lone pair electrons from oxygen (alpha effect) as well as the increased polarity (inductive effect). Since oximes are more stable, they are typically used in applications where greater stability is required, often being used for cell targeting, lectin targeting, and biological imaging. For alternative methods of biological imaging consider checking out our article about bifunctional chelating agents and how they act as a bimodal agent.
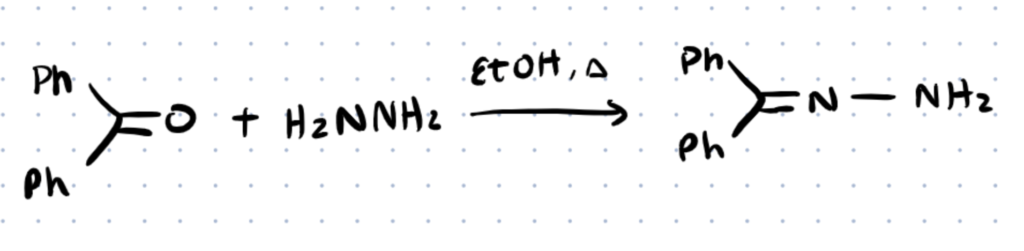
Benzophenone Hydrazone Formation and Uses
Benzophenone hydrazone is not formed as simply as some other hydrazone complexes. In this synthesis Hydrazine and Benzophenone are refluxed in an absolute ethanol medium to form benzophenone hydrazone. Due to the aromaticity of the aryl groups of the benzophenone this hydrozone tends to be very stable due to resonance. Benzophenone has many characteristics that make it valuable for pharmaceutical applications. Benzophenones are known to have high antitumor activity, high selectivity and inhibition of inflammatory cytokines, and they are excellent iron chelators which have applications in malarial medications.
Hydrazine Bioconjugation Techniques
Recent research has investigated hydrazine bioconjugation for many applications including bio-imaging and drug delivery. While the basics of hydrazone formation is well known and documented, many newer techniques are being developed to improve selectivity and performance of the conjugates. The following sections will discuss the protocols for two protocols developed for hydrazine conjugation, one for the bioconjugation of gold nanoparticles for electrochemical sensing of breast cancer tissue and another for conjugation of the ketone group of doxorubicin (DOX) to the hydrazine group of pullulan for a pH sensitive drug delivery system.
Protocol 1. Bioconjugation of Gold Nanoparticles
Here’s a protocol for the bioconjugation of hydrazine to gold nanoparticles used for electrochemical diagnosis of breast cancer. For a more detailed set of steps please refer to the original article (Y. Zhu, P. Chandra, and Y.B. Shim). To learn more about the bioconjugation of nanoparticles check out our linker article.
Step 1. Thiolation of Gold Nanoparticles
First a gold nanoparticle solution must be prepared and then add 2.23 nmol of thiolated aptamer to the mixture and incubate for 12 hr at 4 oC. The resulting solution should contain thiolated gold nanoparticles (Au-S). The excess thiolate aptamer can be removed by centrifuging the solution at 13,000 RPM for 15 min. Collect the precipitate and wash it three times with a phosphate-buffered saline (PBS) solution and the thiolated gold nanoparticles will be ready for the next step.
Step 2. Conjugation of Hydrazine To Nanoparticles
Add the washed thiolated nanoparticles to a 10 mM hydrazine sulfate solution and incubate at 4 oC for 12 hr. Here the hydrazine will ionically bond with the surface of the gold nanoparticles through electrostatic interaction and the mixture can then be centrifuged for another 15 min to remove excess hydrazine. The precipitate is then washed again with PBS and the resulting bioconjugate can be dispersed in a 0.1 M PBS solution.
Protocol 2. Pullulan-DOX bioconjugation
Here’s a protocol for Pllulan-DOX bioconjugation for a pH sensitive drug delivery system. For a more detailed set of steps please refer to the original article (D. Lu et al.).
If your carbohydrate has readily available amines or carboxyl groups, you can use these kits to attach it to other biomolecules like proteins, antibodies, and polymers.
Step 1. Pullulan Carboxymethylation
To produce carboxymethylated pullulan (CMP) first dissolve 5 mg pullulan in 20 mL DI water. Then repeat the following steps 3 times:
- Add 3 mL isopropyl alcohol dropwise to the solution under vigorous agitation at room temperature.
- Add 1 mL sodium hydroxide solution (62% w/v) at 4 oC dropwise to the solution while continuing stirring.
- Heat solution to 70 oC and continue stirring for 15 min.
- Add 3 mL of sodium chloroacetate (60% w/v) dropwise and wait for another 15 min.
After the 3 repetitions, allow the reaction to continue at 70 oC for 6 hours and then allow the solution to cool to room temperature. Next dialyze the solution against DI water for 3 days and freeze dry it to produce a CMP powder.
Step 2. Pullulan Amidation
To introduce amides into the pullulan first add 0.42 ml of 80% hydrazine hydrate to 12 mL DI water and adjust the pH to 5.8 using HCl and then dissolve 2.5 g of CMP powder into the solution. Add 0.26 g of 1-Ethyl-3(3dimethylaminoprpyl)carbodiimide (EDC) to the solution, maintain the pH between 4.5 and 5.8 for 60 min, and repeat this process 3 times. Finally, dialyze the solution against DI water for 2-3 days and freeze dry resulting amidated pullulan (AMP) to obtain a powder.
Step 3. Pullulan/DOX Conjugation and Nanoparticle Formation
To synthesize pullulan/DOC conjugate nanoparticles first dissolve 0.6 g of AMP in 30 mL DI water and add DOX and HCl at a 1 mol ratio (mol DOX/HCL:mol hydrazine). Adjust the pH to 6.0 using 0.2 M acetate buffer and let stir for 24 hours. Next, dialyze the solution against a phosphate buffer (pH 7.4, 10 mM) at room temp for 2 days to allow nanoparticles to spontaneously form. Finally, filter the solution through a 0.45 um filter and freeze dry to obtain a nanoparticle powder. The nanoparticles can be redispersed in a Phosphate-buffered saline solution for further drug synthesis.
Applications of Hydrazine Bioconjugation
Applications of hydrazine bioconjugation include pH sensitive drug delivery systems, bioimaging, and sensing applications.
In the following section these applications will be discussed giving examples with references to recent studies that have investigated hydrazine bioconjugation for each application.
Application 1. pH Sensitive Drug Delivery
Drug delivery is a challenge to modern day science, without a controlled drug delivery system there is no way to ensure that drugs are only being delivered to their needed location. Drug delivery systems are important for patient care and are being investigated extensively to improve drug efficacy, reduce toxicity and side effects, and improve patient response to care.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
There are currently many drug delivery systems available, however many depend on external stimuli such as electrical, mechanical, thermal, and many other stimuli. This requires additional monitoring, intricate systems, and hinders patient care. For these reasons internal drug delivery systems (like those based on pH-triggered cleavage) have been investigated. These systems only depend on natural physiological changes such as pH, enzymatic, and protein responsive systems.
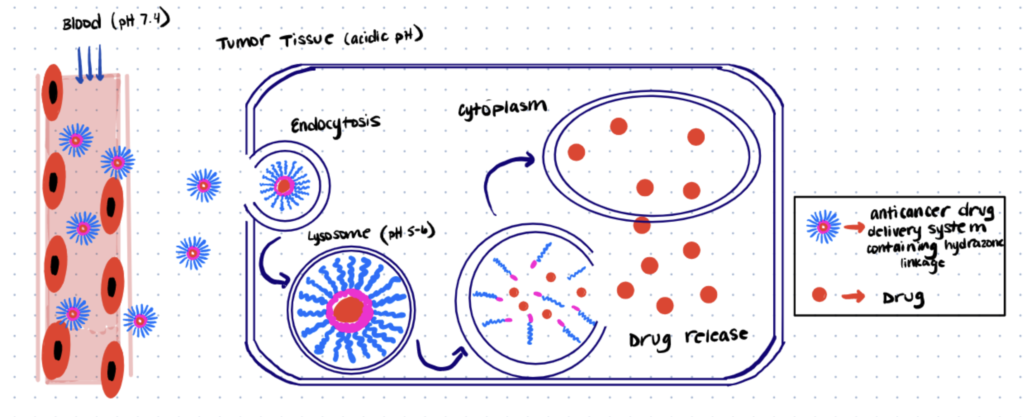
pH sensitive drug delivery systems are constantly being investigated. By controlling delivery using responses to changes in pH, in different areas of the body, patients are able to take a simple pill and receive their treatment. These systems have been used specifically for cancer treatment, based on the findings that lysosomes in tumor tissue have a slightly acidic pH’s (5.0 – 6.0) compared to the bloodstream (pH of 7.4). Hydrazone linkages (as discussed in earlier sections) can be very sensitive to pH and their stability can be modified so they readily hydrolyze under slightly acidic conditions while remaining stable at alkaline pH. By forming hydrazone bioconjugates with polymers, it is possible to encapsulate a drug inside a micelle which will dissociate under the lysosome’s acidic pH upon entering the tumor tissue, where the drug can then do its job. The figure below shows a depiction of one such drug delivery system. One study conducted by Lu et al was able to use a simple synthetic method to create a pullulan/doxorubicin bioconjugate, achieving nanoparticles which showed good stability under alkaline pH and almost 60% drug release under acidic pH.
Application 2. Bioimaging
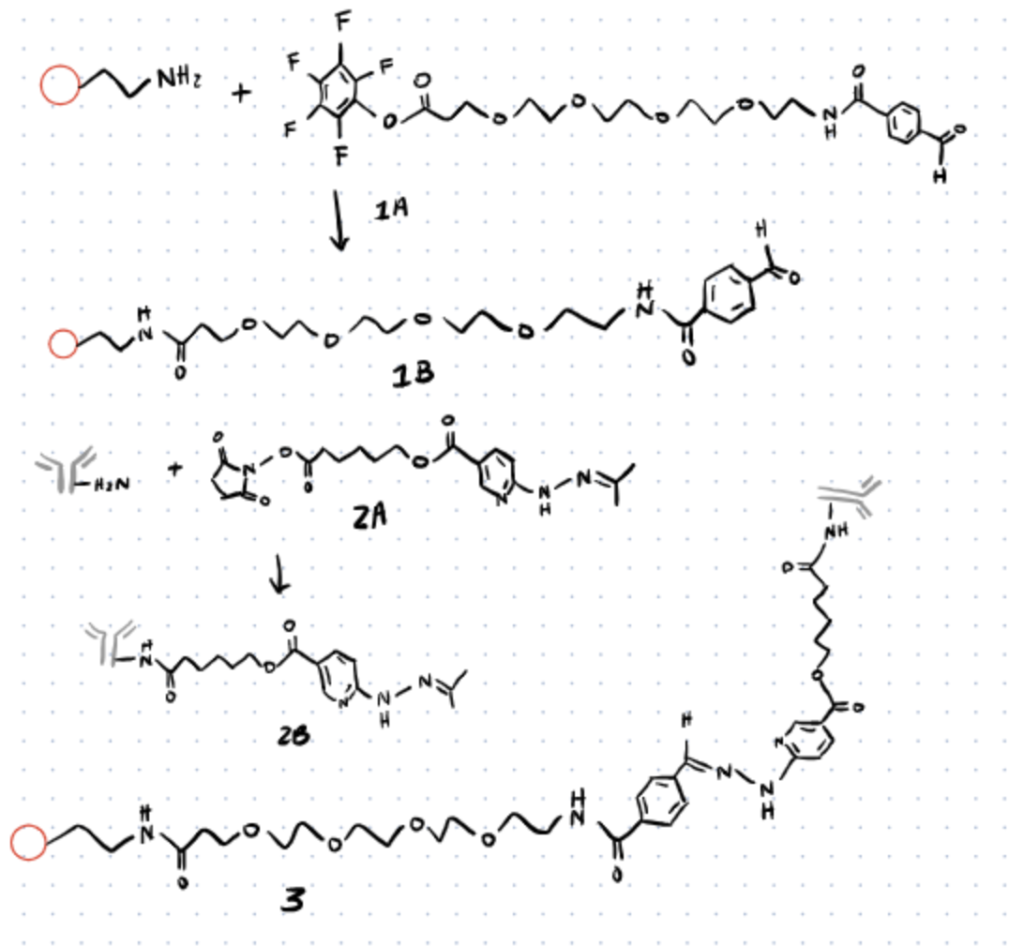
Live cellular imaging is another topic of great importance in modern science. Imaging live cells is not only important for applications such as cancer diagnosis and treatment, but also for studying these cells. Quantum dots are often used for fluorescent cellular imaging due to their size, resonant fluorescence enhancement, and strong fluorescent properties, however these quantum dots often require some bioconjugation to be compatible with biomolecules.
Hydrazine bioconjugates can undergo covalent bis-aryl hydrazone bonds with no side reactions yielding great kinetics. A study conducted by Iyer et al used this to their advantage to create an antibody-quantum dot conjugate system by following the mechanism shown in the image below. Here the peptide coated quantum dot is pegylated to introduce a keto group and the primary amine of the antibody is modified with C6-SANH to introduce hydrazine group with bifunctional linker. The hydrazine of the antibody was then reacted with the keto group of the PEG linker to form a stable bis-aryl hydrazone bond.
After formation, these quantum dot-antibody conjugates could then be incubated with breast cancer tissue. Due to the selectivity of the antibody, the quantum dots were able to be selectively introduced into tissue and imaged using fluorescent microscopy techniques. Consider reading our article about cysteine bioconjugation if you’d like an alternative method for biomaging using site-specific conjugation to cysteines.
Application 3. Biosensing
In addition to bioimaging, biosensing is another area of great interest. Biosensors are sensors of any type that can detect and/or quantify the concentration of a molecule. Biosensors often work on electrochemical or fluorescent techniques and are often characterized by their sensitivity. The ideal biosensing system is simple, portable, and accurate requiring little user interaction, however this is often not achievable when requiring extremely sensitive results. One study done by Zhu et al utilized hydrazine bioconjugation to achieve a simple biosensing system for breast cancer detection. For an alternative biosensing method check out our article about the bioconjugation of maleimide which can be used to build many different biosensors.
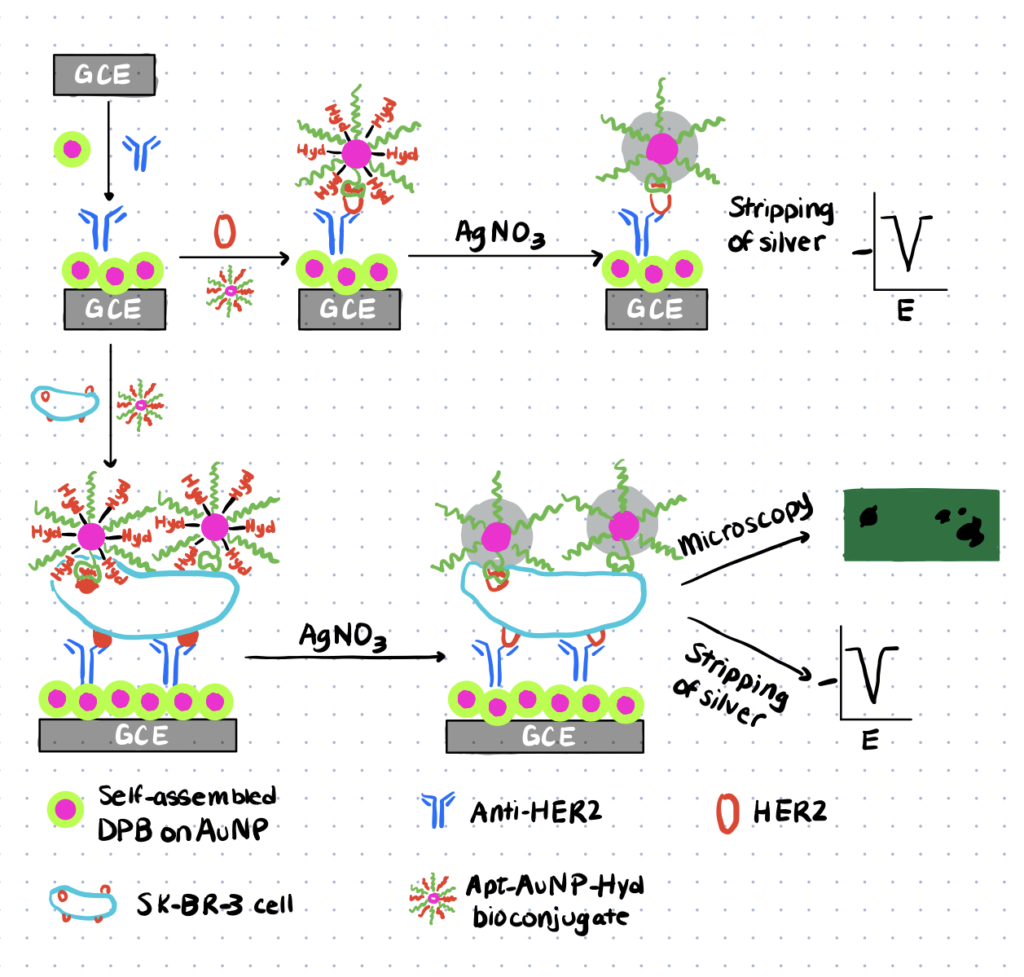
In this study sensitive biosensing and imaging was achieved using silver deposition and silver stripping voltammetry. The image shown below shows the procedure used for this sensing configuration. First the breast cancer antibody immobilized on a glassy carbon electrode that has been coated by 2,5-bis(2-thienyl)- 1H-pyrrole-1-(p-benzoic acid) (DPB) coated gold nanoparticles to produce the sensing device. Next the breast cancer cells were incubated with aptamer-gold nanoparticles conjugated with hydrazine. These particles were formed first by thiolation of the aptamer-gold nanoparticles followed by electrostatic interactions of hydrazine with the gold nanoparticle surface. The aptamer allows for bonding to the cancer cell and after this incubation is complete silver nitrate is added to the system to selectively deposit gold onto the hydrazine surface. Here the hydrazine selectively reduces the silver nitrate ions resulting in silver metal deposition onto the nanoparticles surface. After stripping, voltammetry is applied to quantitatively analyze the amount of silver deposited which can then be related to the concentration of cancer cells in the sample.