It all began from the curiosity of a Hungarian student over some leftover meals. After proving that his landlady was recycling food by spiking his food with radioactive materials, George de Hevesy successfully created a whole territory for nuclear science researchers to exploit. In this article we will discuss the use of bifunctional chelating agents in creating radiolabeled biomolecules.
Bifunctional chelating agents (BFCAs) are molecules containing two functional moieties, one strong metal chelating ligand and one functional group to aid in the formation of a stable covalent bond.
Of the two main strategies for the radiolabeling of macromolecules, indirect labeling is the preferred radiolabeling strategy. Indirect labeling, especially those incorporating bifunctional chelating agents, provides higher stability/control over binding orientation and easier synthesis to enhance the radiolabeling efficiency and pharmacokinetics properties of these naturally decaying atoms (Brechbiel, 2008).
What are Bifunctional Chelating Agents?
The primary prerequisite of radiolabeling is the construction of a stable and well-coordinated link between a potent radioisotope and the carrier biomolecule.
Bifunctional chelating agents contain one functional group for creating a stable bond to a biomolecule, and one chelator that can coordinate with a radiolabel.
The combination of these two modalities allows BFCAs to act as a bimodal agent, either as the visualization source for molecular imaging or to exhibit the cytotoxic effects in radiotherapy. The linker is not directly involved in the complexation but plays an important role in surface stabilization by providing high thermodynamic stability and kinetic inertness between the radioisotope and the carrier molecule (Lattuada et al., 2011; Liu, 2008). For more general information about bifunctional crosslinkers or bioconjugation linkers, check out our linked articles.
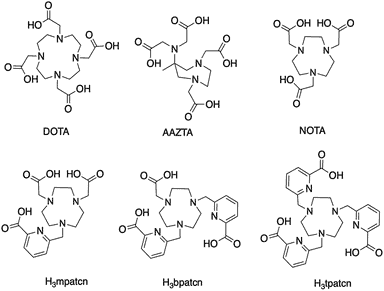
Examples of Common Bifunctional Chelating Agents
Common bifunctional chelating agents utilized in literature include EDTA-based chelators, DTPA-based chelators, and DOTA-based chelators.
1. EDTA-Based Chelators
EDTA, a polyprotic acid compound containing four carboxylic acid groups and two amine groups, is a classic chelator that can chelate many divalent and trivalent metals. EDTA-based chelators are often used in the food industry as a sequestering agent for enzyme cofactors, or just to remove the metal contamination acquired during processing (Versene™).
Calcium disodium salt, also known as calcium EDTA, has also been approved by the FDA for use in treating heavy metal poisoning. However, the use of calcium EDTA needs to be carefully monitored as prolonged use may cause dangerous toxic side-effects due to excessive chelation of essential metals—the most marked being due to the excretion of zinc.
2. DTPA-Based Chelators
The adverse effects of EDTA have forced a move to develop bifunctional DTPA-based chelators containing two amine-reactive anhydride groups. DTPA has similar properties to those of EDTA but forms stronger chelates with transition metals such as Ca, Fe, and Zn.
DTPA chelators have been used for various therapeutic/imaging purposes. In August, 2004 the FDA approved zinc-DTPA and calcium-DTPA to be safe and effective for treatment of plutonium, americium, or curium contamination. DTPA chelating properties are also useful in deactivating Ca/Mg ions in cosmetics, as aquarium plants fertilizer, or to help increase production yield in pulp and paper mills.
3. DOTA-Based Chelators
Despite the successes achieved with DTPA chelators, the instability of the formed complexes under physiological conditions could potentially contribute to toxicity for targeted radiopharmaceutical applications. In response to that deficiency, DOTA chelators containing four nitrogens four secondary amine groups should be the ligand of choice (Woods et al., 2004; Sarka et al., 2000). To learn more about radiopharmaceutical applications, check out our article about bioconjugation methods for radiopharmaceutical chemistry.
DOTA is one of the most potent chelators for theranostics applications. DOTA chelators form complexes with high thermodynamic stability and kinetic inertness. Its complexes have medical applications as MRI contrast agents under the name gadoteric acid, and cancer treatments/diagnosis. Concurrently, a bifunctional DOTA that makes use of one carboxylate in an active ester form for monoclonal antibody conjugation has also been developed and is also a commercially available product to target tumors. You can learn more about antibody conjugation by reading our linked article.
Protocols for Using Chelators
An effective BFCA is expected to complex a radioisotope with limited half-life rapidly/tightly and possess a high kinetic inertness to transchelation by metal cations and natural chelators present. As the functionality/handling of radioisotopes become more complex, chemistry techniques for radiolabeling to optimize their therapeutic potential have also evolved in line (Lattuada et al., 2011; Liu, 2008).
Method 1. Using TCMC-Based Chelators Radiolabeling of Antibodies
Promising preclinical and early clinical studies of 212Pb and its daughter 212Bi have highlighted their potential to help move the fields of radiotherapy and radiodiagnostic forward. Although DOTA is the most commonly utilized class of chelator for Pb(II), studies evaluating the usefulness of DOTA[Pb(II)] complex in vivo have led to conflicting results (Pippin, 1995; Ruegg et al., 1990; Maumela et al., 1995).
Recently, with emerging experiments promoting the superior qualities of TCMC-based conjugates, they have become the preferred chelation chemistry of choice for Pb(II). In contrast to the current ion exchange-based generators, which may be eluted several times, Westrøm et al., (2017) proposed an in situ procedure without subsequent removal of 224Ra as an easier and safer strategy for preparing 212Pb radioimmunoconjugates, as outlined below.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
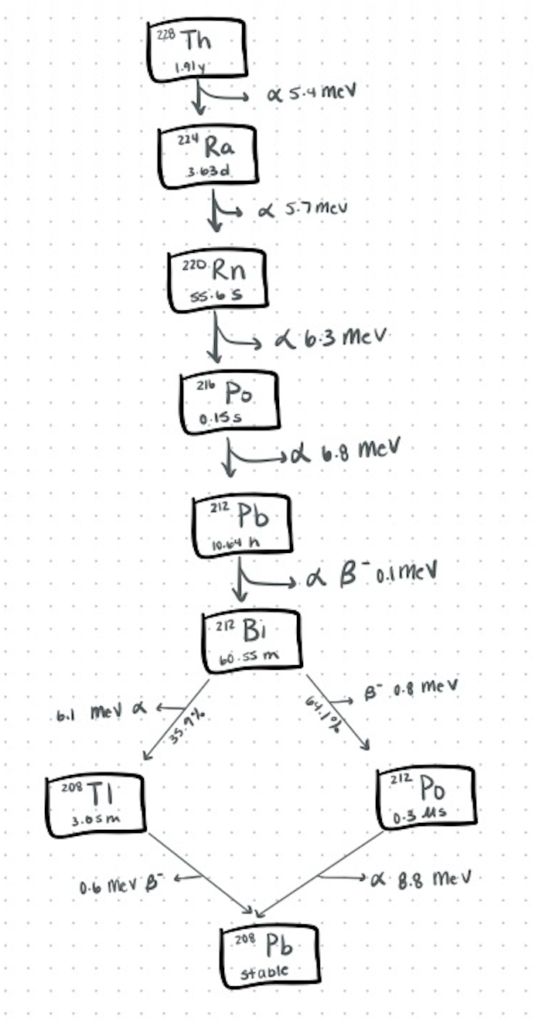
Step 1. The 224Ra-generator
The 224Ra-212Pb solution was first extracted from a generator column containing actinide resin with immobilized 228Th. Measurements of the radioactivity were conducted after the resulting 224Ra solution was left for a minimum of 3 days when the equilibrium had been established. The obtained solution was then used for producing 212Pb-labeled ligands in situ.
Step 2. Radiolabeling of Antibodies
Humanized anti-HER2 IgG1 monoclonal antibody trastuzumab was first conjugated to TCMC. After removal of unconjugated chelator, the solution was then incubated together with the radioactive solution—in which the 212Pb was complexed by the ligands in the presence of 224Ra, yielding a mixture containing 212Pb-TCMC-trastuzumab conjugates, free 224Ra, and 212Bi daughters.
Measurements of the radiochemical purity of the conjugates using ITLC strips yielded a product with RCP above 90%. The immunoreactive fraction of 212Pb-TCMC-trastuzumab was then determined in a one-point, live-cell binding assay and the results were consistent with the immunoreactivity of 212Pb-TCMC-trastuzumab labeled with the method described previously.
Step 3. Purification of radiolabeled antibodies
Purification was done using HPLC and desalting gel filtration column to remove free 224Ra and other unconjugated daughter nuclides. With a yield of approximately 80%, the authors believe that this procedure is beneficial from a logistic, handling and safety point of view compared to other methods generating 12Pb-labeled antibody conjugate conjugates available.
HPLC can also be used to select and quantify bioconjugate products for bioconjugation characterization.
Method 2. H3mpatcn-Based Room Temperature Radiolabeling for Molecular Imaging
Over the past decades, Positron Emission Tomography (PET) has become a practical, high-performance clinical imaging modality for visualization of biological processes in vivo. Among the few metal-based PET radioisotopes available, DOTA[Ga(III)] are of particular interest.
However, although DOTA forms very stable complexes, complex formations are slow at room temperature due to the kinetic inertness of the macrocycle. Heating at 70–95oC can increase the kinetics to achieve adequate yields/activities but highly limits its applications to heat-resistant targeting vectors such as antibodies (Tircsó et al., 2020; Mukai et al., 2009).
Inspired by lanthanide chemistry, Vaughn et al. (2020) proposed the use of 44Sc for this purpose. Using a novel triazomacrocycle based chelator functionalized with picolinic acid (called H3mpatcn). The team successfully validated the tracer represent excellent additions to the chelator toolbox for the emerging 44Sc as a theranostic isotope for PET.
Step 1. Ligand design
The H3mptcn ligand was designed using lanthanide-chemistry based design principles and was screened with cyclotron-produced 44Sc using a medicinal inorganic chemistry approach. The NMR spectroscopic and radiochemical studies showed that the [Sc(mpatcn)] complex remains kinetically inert under radiochemical and physiologically relevant conditions.
Step 2. Radiolabeling with 44Sc
Based on the promising results obtained with the H3mptcn-based chelator, the authors synthesized a proof-of-concept experiment incorporating picaga-DUPA, a functionalized mpatcn derivative synthesized through alkylation. Following complete characterization, the picaga-DUPA conjugates were subjected to time/temperature-dependent radiochemical complexation experiments, with DOTA-DUPA conjugates as a reference.
The results showed that the 44Sc(picaga)-DUPA conjugates produced high radiochemical yields, with 83% complexation was achieved at room temperature and 96% at 80oC—comparing favorably to radiolabeling of 44Sc with DOTA-conjugates at 95oC. The authors hypothesized that the efficient structural preorganization of the functionalized derivative accelerates room temperature complexation.
Step 3. PET imaging and Biodistribution Studies
In vivo imaging and biodistribution study of the 44Sc(picaga)-DUPA compared well to the clinically validated metal-based PSMA-inhibitor, MIP-1427. This demonstrates the efficacy of the conjugates for future clinical translation and is suitable for tandem radiotherapeutic applications with 44Sc.
Method 3. Bimodal Functionalization of Quantum Dots Using Chelators
Quantum dots (QDs) possess unique properties that make them ideal for the development of nano-theranostics platforms for targeted applications. Functionalization of QDs have mostly been prepared using direct approaches. However, for clinical applications such as MRI contrast agents (Gd(III)-QDs), direct approaches offer some limitations to extracellular applications at molecular levels (Verwilst et al., 2011). We’ve covered bioconjugation of quantum dots in detail, in another article.
The drawbacks of this strategy have led to the exploration of experimental approaches to create bimodal probes by combining optical reporter (transition metal) with a different high-sensitivity imaging modality (fluorescence) for improved clinical diagnostic approach. Stasiuk et al. (2011) proposed a new bimodal QD platform with high Gd(III) payload and bright luminescence properties using a novel H3bpatcn chelator described in their previous research.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Step 1. Preparation Of Gd-QD Conjugates
The Gd(bptcn) chelator was prepared through a multistep synthesis and the QDs were synthesized following the protocol reported earlier. The two solutions were then incubated together in an aqueous solution overnight for conjugation of the Gd(bptcn) complexes to the QDs surfaces.
The formed Gd-QD conjugates contained a high payload of gadolinium (70-80) per QD. As indicated by the NMR profile and photophysical analysis, the high-surface area-to-volume ratio of formed complexes provided an architecture with high relaxivity and bright luminescence in water that allows for further functionalization with other imaging agents.
Step 2. Preparation Of Bimodal QD Probes
For further functionalization to produce bimodal QD probes, the authors introduced the peptide maurocalcine in a one-pot reaction to afford Gd.QD.MCa, a cell-penetrating multimodal multimeric MRI contrast agent containing a gadolinium payload in the range of 30-40 complexes per QD
Step 3. Bimodal QD Characterization
To assess the cell-penetrating ability of the Gd.QD.MCa bimodal probes, the authors conducted a confocal microscope and in vivo MRI imaging study. The results indicated that conjugates showed a high potential as not only a dual signal for positive contrast agent, but also a bimodal probe that can penetrate cells and allow for imaging of disease states at different scale lengths and different tissue depth. The conjugates also exhibited better relaxivity in comparison to the commercial CAs used clinically, such as Dotarem ([Gd(DOTA)]).
New Bifunctional Chelators In Development
In the past 2 decades, expansion in the knowledge of nuclear and chemical science has revealed an array of novel BFCAs that are being harnessed for the design of new therapies and imaging biomarkers.
Bifunctional chelators in development include HOPO-based chelators, DFO-based chelators, and a novel FCD-based compound.
Li et al. (2021) investigated the strong chelation application value of newly discovered 3,4-HOPO chelator to bind 177Lu-labelled Cetuximab. This presents a new alternative for radioimmunotherapy of tumours. Intravenous injection of the novel bidentate bifunctional chelating agent, 177Lu-3,4-HOPO-Cetuximab showed a potent anti-tumor effect by decreasing the tumor cell activity and metabolic capacity after 20 days.
For many years, deferoxamine (DFO) has remained the ‘gold standard’ for radiolabelling of 89Zr. However, the instability of DFO-89Zr complexes can lead to unwanted uptake in bones (we’ve covered bioconjugation and cellular uptake in more detail in the linked article). To optimize this chelation chemistry, Chomet et al. (2021) designed two novel chelators—namely the DFO*-NCS and DFOSq—that give more stable 89Zr complexes, exhibit lower bone uptake, and provide superior detection of tumor-specific signals over the conventional DFO conjugates in pilot experiments.One study exploited the potential of fusaricide, a natural compound extracted from fungus to bind intracellular iron. For the first time, Hui et al. (2021) discovered that fusaricide potently inhibited proliferation in cancer cells by capturing intracellular iron—thereby inducing apoptosis, causing DNA damage, and up-/down regulated several cancer related signaling pathways.
