Conjugation techniques depend on two interrelated chemistries: functional groups present on the target macromolecules to be modified and functional groups of the cross linkers used to bind them. Without the availability and chemical compatibility of both types of functional groups, the process of derivatization would be impossible. We discuss different protein protein conjugation techniques in this article using a variety of functional groups.
Protein protein conjugation relates to methods that are used to covalently link two proteins to each other. Generally, crosslinkers bind to functional groups on each protein to generate the covalent links. Protein protein conjugates can be utilized for a variety of applications including western blots, ELISAs, and immunotoxins for drug delivery.
Generally, Protein protein conjugation is carried out by using crosslinkers.
Crosslinking is the process of chemically joining two or more molecules by a covalent bond. Crosslinking reagents (or crosslinkers) are molecules that contain two or more reactive ends capable of chemically attaching to specific functional groups (primary amines, sulfhydryls, etc.) on proteins or other molecules. These crosslinkers can be homo bifunctional or hetero bifunctional in nature. We’ve discussed hetero- and homo- bifunctional linkers in our article on protein conjugation chemistry.
Conjugates are often prepared by attachment of an enzyme, fluorophore, or another molecule to a protein that has an affinity for some other biological molecule. For example, enzymes such as alkaline phosphatase and peroxidase coupled to primary and secondary antibodies are among the most widely used protein protein conjugations.
There are a lot of methods available for protein protein conjugation. A schematic diagram of simple protein protein conjugation is given below.
Related articles:
- How do you bioconjugate proteins to pegylated nanoparticles? Learn more in our article
Applications of Protein-Protein Conjugates
Applications of Protein-Protein conjugates include the study of protein interactions, creating multifunctional enzymes by linking two or more proteins together, or make drug delivery conjugates such as immunotoxins.
The techniques discussed in this article can also be used for protein-small molecule or protein-fluorophore chemistry. For example, you can utilize amine-, carboxyl- or sulfhydryl- reactive reagents to identify and quantify particular amino acids or for determination the number, location, and size of subunits. You can also experiment with different lengths of otherwise identical cross linkers to reveal the molecular distance between particular functional groups in the secondary, tertiary, or quaternary protein structure.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
ELISAs (Enzyme Linked Immunosorbent assays)
ELISAs are a plate-based assay technique designed for detecting and quantifying soluble substances such as peptides, proteins, antibodies, and hormones. Other names, such as enzyme immunoassay (EIA), are also used to describe the same technology.
In an ELISA, the antigen (target macromolecule) is immobilized on a solid surface (microplate) and then complexed with an antibody that is linked to a reporter enzyme. Detection is accomplished by measuring the activity of the reporter enzyme via incubation with the appropriate substrate to produce a measurable product.
The most crucial element of an ELISA is a highly specific antibody-antigen interaction. We’ve discussed ELISA protocols and techniques in detail before.
Western Blotting
Western blotting is an important technique used in cell and molecular biology. By using a western blot, researchers are able to identify specific proteins from a complex mixture of proteins extracted from cells. Western blotting is often used in research to separate and identify proteins.
In this technique, a mixture of proteins is separated based on molecular weight through gel electrophoresis. These results are then transferred to a membrane producing a band for each protein. The membrane is then incubated with a primary antibody specific to a protein of interest. The unbound antibody is washed off leaving only the bound antibody to the protein of interest. The bound antibodies are then detected by binding a secondary antibody that has a reporter enzyme (similar to ELISAs) and the reporter is detected on film. Here’s our detailed guide to Western blotting methods and protocols.
As we discussed above, the secondary antibody used in Western Blotting requires a reporter enzyme to be conjugated to an antibody. This protein-protein conjugation is typically carried out by the antibody manufacturer but you could do it yourself too!
Immunotoxins for Drug Delivery
Specific antibodies can be covalently linked to toxic molecules or proteins and then used to target antigens on cells – these are called immunotoxins. Often antibodies utilized for immunotoxins are specific for tumor-associated antigens.
Immunotoxins are brought into the cell by surface antigens and, once internalized, they proceed to kill the cell by ribosome inactivation or other means.
The type of crosslinker used to make an immunotoxin can affect its ability to locate and kill the appropriate cells. For immunotoxins to be effective, the conjugate must be stable in vivo. In addition, once the immunotoxin reaches its target, the antibody must be separable from the toxin to allow the toxin to kill the cell. Thiol-cleavable, disulfide-containing protein conjugates have been shown to be more cytotoxic to tumor cells than non-cleavable conjugates.
Types of Chemical Handles on Proteins for Protein-Protein Conjugation Chemistry
Over time, proteins have become the most valuable biomolecules among the vast variety of cellular components. Since proteins have high commercial value due to their use in medicine, chemists have been developing many new methods of modifying proteins. Enhanced bioavailability, fluorescent tracking, post-translational modification (PTM) insertions, and targeted delivery are just a few of the numerous possible applications of protein-protein conjugates.
Chemical handles on proteins for protein-protein conjugation include the N-terminus, C-terminus, Lysine amino acids, and cysteine amino acids. Different crosslinking reagents can be utilized to bind to each of these functional groups.
The ability to retain protein function is primarily affected by the site and size of the protein modification. Various chemical handles are used to accomplish successful protein-protein conjugation, some of which are discussed below.
Note: You might be interested in learning about methionine bioconjugation techniques as well!
N-Terminus Protein Conjugation
The N-terminus is the first part of the protein that exits the ribosome during protein biosynthesis. N-terminal α-amines can be either neutral or positively charged depending on the solution pH and chemical modifications. We’ll discuss how to react with amines on proteins later in this article.
N-terminal α-amines are excellent chemical handles for protein conjugation reactions because they are easily accessible and reactive.
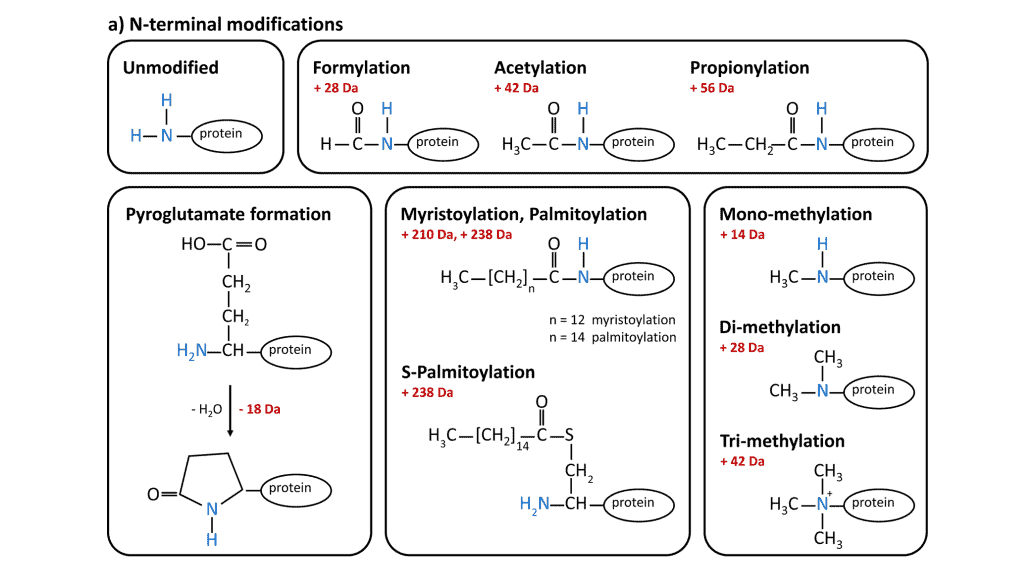
The most prominent protein terminal modification is N α-acetylation catalyzed by N-acetyl-transferases that also have the ability to formylate and propionylate protein N-termini.
Pyroglutamate, commonly observed in antibodies and making them more resistant to aminopeptidases, forms through cyclization of N-terminal glutamine or glutamate, either spontaneously or enzymatically by glutaminyl cyclases. Palmitoylation and myristoylation can occur at free N-terminal glycines, and N-terminal cysteines can be palmitoylated (S-palmitoylation) at their side chain.
Finally, N-terminal mono-, di- and tri-methylation modifications are important for protein-protein interaction.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Chemical Modification of the C-Terminus of Proteins
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH).
The C-terminus contains an accessible carboxyl group which can be used for protein protein conjugation reactions.
The C-Terminal of a protein can be modified at the pre-transitional level based on genes expressed. The C-Terminal is also susceptible to post-translational modification via phosphorylation, glycosylation,acetylation etc.
C-terminal α-amidation neutralizes the negative charge of the carboxyl group at the C-terminus, improving stability and receptor binding ability of peptides and peptide-hormones.
The lower reactivity of the C-terminus, in comparison to the N-terminus, is a reason why C-terminal modifications are rarer. Low reactivity can be one common problem with protein bioconjugation techniques.
In general, you should try to modify proteins at the N- or C- terminus because they are more available, reactive, and don’t need any genetic engineering to include into a protein. Here’s a more detailed article we’ve written about protocols for C-terminal bioconjugation.
Protein-protein Conjugation Using Lysine Residues
Lysine amino acids on proteins are widely used for conjugation chemistry because they contain reactive primary amines.
These can react with activated esters, sulfonyl chlorides, isothiocyanates, 2-amino-2-methoxyethyl and squaric acids, as well as undergo reductive alkylation using aldehydes in the presence of sodium borocyanohydride.
Generally, Lysines are naturally abundant on the surface of proteins. This makes site-selective modification targeting a particular Lys more difficult with conventional modification methods.
On the other hand, if your protein has an abundance of Lysine residues, modifying them can also lead to product heterogeneity because only some of them will be modified.
So, reactions targeting Lys residues as part of protein protein conjugation techniques yield a mixture of heterogeneously modified products.
Related articles:
- You can use lysines to conjugate proteins to surfaces as well! Learn more about surface bioconjugation techniques.
- For a more site-selective method to react with amines is to utilize transglutaminase-mediated bioconjugation. We’ve provided protocols and details in our article.
Utilizing Cysteine Disulfide Bonds For Protein Conjugation
You can utilize the high nucleophilicity of thiol groups on cysteine amino acids to chemically modify proteins.
Most cysteine residues in proteins are oxidized as part of disulfide bonds and give the protein structural stability.
In general, chemists utilize maleimides to react with thiols on cysteines because the reaction is efficient and selective. However, some more novel reagents with thiol-specific reactivity are also being developed, as we describe in the picture below. These reactions lead to irreversible, stable conjugates and are as efficient as maleimide reactions. We’ve covered cysteine bioconjugation techniques and protocols in detail in another article.
For Michael addition, the cysteine amino acid can also be converted to dehydroalanine (Dha) to introduce an electrophilic handle.
You cannot easily modify proteins using their cysteine residues if the cysteines participate in catalytic function for the protein or if they are involved in protein folding or structural stability.
Related articles:
- Using orthogonal bioconjugation techniques, you can react with multiple handles on proteins
Protein-Protein Conjugation Techniques
Protein-Protein conjugation is performed with the help of cross linkers which can be either homobifunctional or heterobifunctional.
As an example, if two proteins contain sulfhydryls, homobifunctional sulfhydryl crosslinkers may be used to couple them. Other homobifunctional cross linkers such as NHS-esters or imidoester may also be used.
Homobifunctional cross-linkers can potentially self-conjugate and polymerize. It’s also possible for excessive self-conjugation to lead to the formation of insoluble complexes that consist of very high-molecular-weight polymers.
Heterobifunctional cross-linkers, on the other hand, do not pose the risk of self-conjugation and are a great choice for antibody-enzyme and other protein-protein conjugations.
There are a wide variety of techniques which are used to form protein-protein conjugates.
Glutaraldehyde-Mediated Conjugation
Glutaraldehyde was one of the first crosslinking agents and it is still one of the most commonly used crosslinking agents available for creating antibody–enzyme conjugates.
To use glutaraldehyde for antibody-enzyme conjugation, the typical mechanism involves Schiff base formation with possible rearrangement to a stable product or through a Michael-type addition reaction.
In aqueous solutions at alkaline pH, glutaraldehyde has a possibility to form a large polymer structure through aldol condensation reactions with itself containing α,β -unsaturated aldehydes. Another disadvantage of the reagent is the tendency to form high-molecular-weight conjugates due to uncontrollable polymerization during the crosslinking process. The resultant conjugates often have a significant amount of insoluble polymer which causes yield and activity losses in the preparation of antibody–enzyme conjugate.
Reductive Amination-Mediated Conjugation
To utilize reductive amination-mediated protein protein conjugation or antibody protein conjugation, you need at least one glycoprotein. First, oxidize carbohydrate moieties in your glycoprotein. Then, couple the aldehydes on your carbohydrate to an amine on your other protein target.
This procedure is called reductive alkylation or amination. These conjugates often result in less background in enzyme immunoassays and are relatively easy to prepare. However, some self-conjugation of the antibody may occur.
To oxidize the polysaccharide on your glycoprotein or antibody, treat it with sodium periodate. The resulting aldehydes on your protein can easily be coupled to amines or hydrazide-containing molecules via reductive amination.
You can selectively choose which carbohydrate to oxidize by regulating the concentration of periodate in the reaction medium.
NHS Ester–Maleimide-Mediated Protein Protein Conjugation
Heterobifunctional reagents containing an amine-reactive NHS ester on one end and a sulfhydryl- reactive maleimide group on the other end generally have great utility for producing antibody–enzyme conjugates. Crosslinkers containing these reactive groups can be used in highly controlled, multi-step procedures which yield conjugates of defined composition and high activity. Most widely used crosslinkers of this type are SMCC and its water-soluble analog, sulfo-SMCC which possess most stable maleimide functionalities. This increased stability to hydrolysis of SMCC ’s hindered maleimide group allows activation of either enzyme or antibody via the amine-reactive NHS ester end, resulting in a maleimide-activated intermediate. The intermediate species then can be purified away from excess crosslinker and reaction by-products before mixing with the second protein to be conjugated. Due to the multistep nature of this process polymerization of the conjugated proteins decreases in a large extent and provides control over the extent and sites of crosslinking.
Reduction of the disulfide bonds within the hinge region of an IgG molecule produces half-antibody molecules containing thiol groups. Reaction of these reduced antibodies with a maleimide-activated enzyme creates a conjugate through thioether bond formation.
Conjugation with SATA-Modified Antibodies
N-Succinimidyl-S-acetylthioacetate (SATA) is a thiolation reagent which reacts with primary amines via its NHS ester end to form stable amide linkages. The acetylated sulfhydryl group is stable until deacetylated with hydroxylamine. Thus, antibody molecules may be thiolated with SATA to create the sulfhydryl target groups necessary to couple with a maleimide-activated enzyme.
Using this reagent, stock preparations of SATA-modified antibodies may be prepared and deacetylated as needed. Unlike thiolation procedures which immediately form a free sulfhydryl residue, the protected sulfhydryl group of SATA-modified proteins is stable to long-term storage without degradation.
Available amine groups on an antibody molecule may be modified with the NHS ester end of SATA to produce amide bond derivatives containing terminal protected sulfhydryls. The acetylated thiols may be deprotected by treatment with hydroxylamine at alkaline pH. Reaction of the thiolated antibody with a maleimide-activated enzyme results in thioether crosslinks.
Immunotoxin Conjugation Techniques
The basic design of an immunotoxin conjugate consists of an antibody-targeting component crosslinked to a toxin molecule. This technique includes a disulfide bond between the antibody portion and the cytotoxic component of the conjugate which allows release of the toxin intracellularly. In this illustration, an intact A–B toxin protein provides the requisite disulfide.
The B chain contains a binding region for docking onto cell surfaces, while the A chain contains a catalytic site that produces cytotoxic effects intracellularly. The two subunits are joined by a disulfide bond that is reductively cleaved at the cellular level and after this cleavage the A subunit causes cells death.
Immunotoxin conjugates consist of an antibody covalently crosslinked to a toxin molecule in a way that unique properties of both proteins are maintained. The antibody component consists of a monoclonal having specificity for an antigenic determinant on the surface of a particular cell type. Most often, the targeted cells are tumors that express a unique cell-surface marker which can be recognized by the monoclonal. The antibody works like a passive taxi, carrying the toxin component to the targeted cells. When it reaches the tumor location, the toxin component affects its intended ribosome, ultimately causing cell death and tumor destruction.
Besides antibodies, you can also conjugate toxins to carbon nanotubes for tumor targeting via the EPR effect.
