Western blotting is a method to detect proteins in biological samples and was first described by researchers more than four decades ago. Today, it has become the most popular method for accurately detecting proteins–almost every biology lab in the world relies on this important technique for their research. Although technically demanding, western blotting is useful in determining the presence (or absence) of proteins, the abundance of a protein relative to another, protein localization, protein-protein interactions, and even the post-translational modification state(s) of a protein. Therefore, western blot is an indispensable yet versatile tool that allows researchers to study protein biology, but also proteomic changes caused by diseases and injuries, and even evaluate efficacies of drugs and therapeutics. In this article, we will discuss techniques and protocols for optimization of western blot exposure time and detection.
A successful western blot is characterized by optimized detection and imaging methods that allow for the accurate readout of proteins-of-interest. To choose western blot exposure time for chemiluminescence detection with an x-ray imager, start with a 1-minute exposure time and adjust accordingly to avoid overexposure.
Western Blot Fundamentals And Technical Challenges
A western blot involves protein separation, membrane blotting, immunodetection, and image acquisition. Detection and imaging are steps in the workflow that need to be carefully optimized.
As an antibody conjugation method for the immunodetection of proteins-of-interest, a western blot involves four key steps:
Electrophoresis
A pool of proteins in a sample is separated based on its molecular weight. This is done by running the sample on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. At the end of this step, you will obtain a well-resolved gel containing proteins.
Blotting
Transferring of the proteins from the SDS-PAGE gel to a piece of nitrocellulose membrane via the process of electrophoretic transfer. The nitrocellulose membrane serves as an immobilization surface for the proteins.
Immunodetection
Proteins on the nitrocellulose membrane are then probed using primary antibodies that specifically bind to the proteins-of-interest. Secondary antibodies that recognize the primary antibodies will then be added. The type of secondary antibodies used will ultimately depend on the method of image acquisition that you wish to utilize. For example, one could utilize FITC labeling to add FITC to a secondary antibody and image it in a fluorescent western blot.
Image Acquisition
Western blot images can be acquired by two modes – chemiluminescence and fluorescence. Chemiluminescence-based imaging can be further divided into digital or X-ray imaging while fluorescence-based imaging is performed digitally.
Although the western blotting technique is both popular and easily accessible, and the technique has been refined and improved tremendously since its conceptualization, 41% of researchers reported that one in four of their western blot experiments fail (Liu, 2014). A recent review paper aptly summarized the technical challenges and considerations associated with western blotting (Bass et al., 2016). Western blot clearly remains to be relatively challenging assay to perform. Why is this so? In contrast to high selectivity and rapidness of antibody-biotin conjugation methods for protein detection, western blotting is a multi-step process and has numerous variables. These variables have to be carefully optimized with thoughtful considerations of the nature of the proteins, types of antibodies used as well as the different image acquisition methods as mentioned above.
In this article, we will be exploring more on the detection and imaging of ECL western blots, comparing chemiluminescence-based x-ray film imaging with fluorescence-based digital imaging. Lastly, we will be discussing factors to consider when selecting exposure times in order to improve the quality of your western blots.
What Are ECL Western Blots?
Using ECL substrate and HRP secondary antibodies for western blots, in addition to x-ray film or digital imaging, improve the detection of proteins-of-interest.
Enhanced Chemiluminescence (ECL) is a western blotting substrate that is a sensitive, stable, and cost-effective horseradish peroxidase (HRP) substrate. It is also highly compatible with nitrocellulose membranes (Mruk and Cheng, 2011). HRP is one the most popular enzymes used in western blotting and, through protein-protein conjugation techniques, is typically conjugated with the secondary antibodies that recognize and bind to primary antibodies. In the presence of luminol in the ECL substrate and peroxide buffer, HRP catalyzes a reaction that produces chemiluminescent signals. Chemiluminescence is a chemical reaction whereby energy is released in the form of light. The light signals are then captured and acquired. The presence of the chemiluminescent signals signifies the presence of the protein that is being probed for and vice versa.
One of the most attractive features of ECL western blots is that the chemiluminescent signals can be detected via the use of X-ray films or digitally using a detection system. Another feature of ECL western blot is that under optimal conditions, the chemiluminescent signals can last for 6-24 hours, depending on the type of ECL substrate. However, a considerable downside of ECL western blot is that high amounts of HRP (such as if the protein that is being probed for is highly abundant) will produce high amounts of free radicals during the chemical reactions, which can damage the nitrocellulose membrane. However, this issue could be resolved by reducing the amount of primary antibody used during the immunodetection step or by reducing the amount of protein samples loaded into the SDS-PAGE gel.
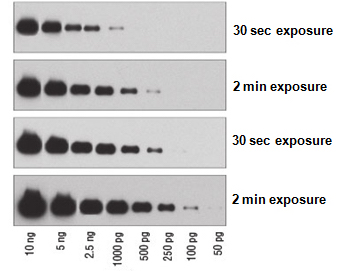
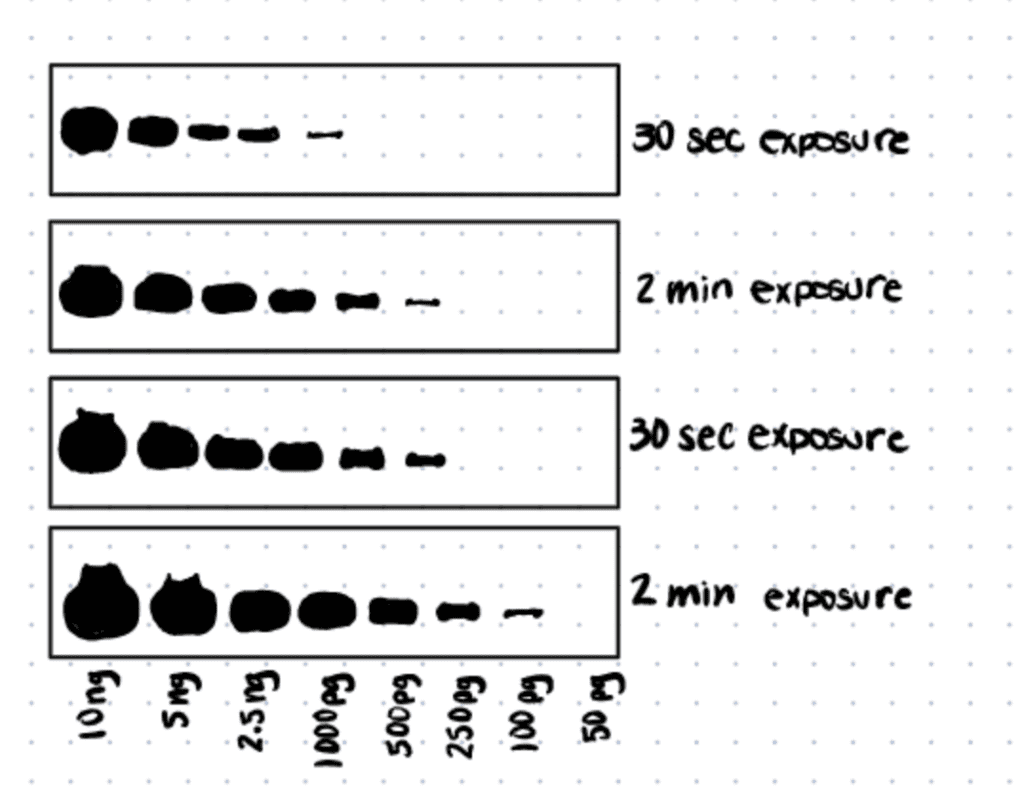
Western Blot Imaging Techniques
Proteins-of-interest probed through western blot can be detected by x-ray film imaging, which appear as dark bands and is sensitive to chemiluminescent signals, or digital imaging using a CCD imager that converts emitted photons into digital signals.
Technique 1. X-Ray Film Imaging
X-ray film imaging is arguably the most sensitive method to detect proteins on western blots. It has long been referred to as the ‘gold standard’ of sensitivity for western blot imaging (Bio-Rad). The X-ray film contains substrates that undergo chemical changes in the presence of light. When exposed to a chemical developing solution during the film developing process, the reaction forms a dark image. The light emitted from the HRP-ECL chemical reaction can easily be captured by the X-ray film. When developed, these signals produced dark bands on the X-ray film. The entire process of exposing and developing the film has to be done in a dark room.
The reason why X-ray film is sensitive is because the film is in close physical proximity to the blot that is emitting light signals due to the HRP-ECL chemical reaction (Bio-Rad). However, the protein bands or signal bands are semi-quantitative since X-ray film has a limited dynamic range (ThermoFisher Scientific). The film is easily saturated by moderate to high signal intensities. Additionally, X-ray films and film developing solutions are typically expensive.
Technique 2. Digital Imaging
Alternatively, western blots can be digitally imaged using a Charge-coupled Device (CCD) imager, which converts photons into digital signals. Essentially, a CCD functions as a large, sensitive, and automated camera that detects the signals produced on the blots and captures them. This allows the acquisition of western blot images to be convenient since it does not involve the use of physical pieces of X-ray films and does not have to be performed in a dark room.
2.1 Western Blot Ecl Detection
ECL western blots can also be detected and imaged via digital imaging. The CCD imagers capture chemiluminescent signals produced by the HRP-ECL chemical reaction and quantify the signals. As compared to the close proximity of the X-ray films to the blots, when using the CCD imager, light signals have to travel from the blot to the detector. Therefore, X-ray films are more sensitive than CCD imagers (Cytiva, 2018). However, with the advancement of technology, the sensitivity of CCD imagers has progressively improved in recent years. The broad dynamic range of CCD images also allows the detection of both low and high signal intensities.
2.2 Western Blot Fluorescence Detection
Digital imaging is also used for fluorescent western blots. Instead of HRP-conjugated secondary antibodies, fluorescent western blots utilize fluorophore-conjugated secondary antibodies. These fluorophores are excited upon exposure to different wavelengths of light, resulting in the emission of photons that are captured by the CCD imager. Unlike ECL western blots, fluorescence western blots allow for the detection of more than one protein simultaneously. Fluorescent western blots are convenient since it only involves a one-step imaging process for the detection of more than one protein. The amount of fluorescence is directly proportional to the amount of proteins on the membrane. Therefore, the proteins/ signal bands detected by the CCD imager signifies the quantities of the proteins-of-interest.
Below is a table comparing x-ray film Imaging with ECL and digital imaging with fluorescence.
| X-Ray Film Imaging with ECL | Digital Imaging with Fluorescence | |
| Sensitivity | Highly sensitive (femtogram range), especially for proteins that are in low abundance | Sensitive. Variable between detection systems. |
| Speed | The speed at which the X-ray film is exposed to the light emitted from the ECL chemical reaction is very fast. However, the speed by which the films are developed depends on the film processing machines, which vary depending on model and age. | The speed at which the fluorescent signals are detected and captured by the digital detection system is fast. |
| Signal-to-noise ratio (specificity) | Since X-ray films are highly-sensitive, the signal-to-noise ratio is reduced. | High signal-to-noise ratio due to advanced and high-resolution cameras. |
| Dynamic Range | Limited | Broad |
| Efficiency of changing exposure times | Less efficient. A new piece of X-ray film will have to be replaced in a dark room for every exposure time. | Efficient. Different exposure times can be tested easily. |
| Quantitativeness | Semi-quantitative. Protein bands on the X-ray films can be scanned into an image file and quantified using software. | Quantitative. Most detection systems are equipped with software that can readily quantify the western blot signals |
| Compatibility to fluorescence western blots | Not compatible | Compatible |
| Probing multiple proteins | Cannot be done | Can be done |
| Documentation | Images are saved as physical copies of the X-ray films. | Images are saved and documented digitally. |
Western Blot Exposure Time Considerations
Western blot exposure time for protein detection depends on the saturation of total protein, abundance of proteins-of-interest, and the concentration and binding affinity of antibodies used for immunodetection.
Western blot long exposure times will result in over-saturation of chemiluminescent or fluorescent signals while short exposure times will result in insufficient/ faint signals. The key question that many researchers have is how does one find the optimal exposure time to detect the proteins-of-interest? Typically, if you are detecting a protein or using a new antibody for the first time, it is recommended to start with one minute exposure time. Then, increase or decrease accordingly based on the amount of signal obtained.
When considering western blot exposure time, there are three important factors to take note of:
Amount Of Protein Loaded Into The Gel
The higher the amount of protein loaded into the SDS-PAGE gel, the higher the abundance of the proteins-of-interest. Therefore, a lesser exposure time is required to detect them. Therefore, if a large amount of protein is utilized in preparing the western blot, a shorter exposure time can be used as a starting exposure time.
For more information on proper loading procedures and amounts, see our article, how to choose western blot loading amounts.
The Abundance Of Proteins-Of-Interest
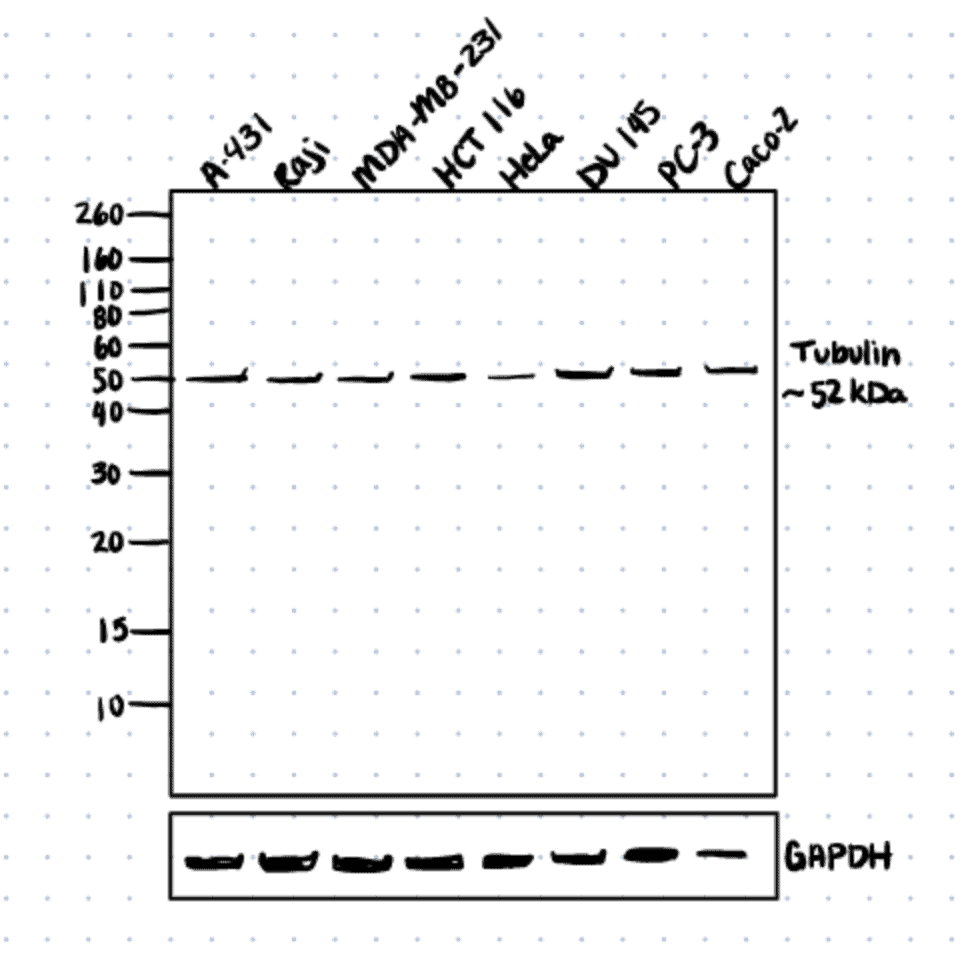
Similar to the point above, the higher the abundance of the proteins-of-interest, the lesser exposure time is needed to detect them. For example, housekeeping proteins such as GAPDH and β-Actin are typically in high abundance, therefore, a short exposure time (<30s) is needed to detect them on both X-Ray films or CCD imagers.
Concentration And/Or Binding Affinity Of Antibodies
If a high concentration of the primary antibodies is used in the immunodetection step, more primary antibodies will bind to the proteins-of-interest. In turn, more conjugated secondary antibodies will bind to the primary antibodies. This will result in a higher amount of signal (due to the presence of more HRP enzyme or fluorophore) and thus, shorter exposure time.
Read our article, how to choose the best western blot negative control to improve western blot results when determining exposure times.
X-Ray Film Western Blot Protocol
To perform x-ray film imaging of immunolabeled western blots, transfer the blot onto saran wrap in a film cassette with ECL substrate mixture. In a dark room, place a piece of x-ray film into the cassette and begin with a 1-minute exposure time, then adjust accordingly based on the protein band intensities before developing and imaging.
Below we discuss a general protocol for X-Ray Imaging of ECL western blots. For a more detailed protocol, refer to this article.
Step 1. Place Blots On Saran Wrap In The Film Cassette
Place a piece of saran wrap in the film cassette. Carefully pick blots that have been probed and washed using forceps and remove excess wash buffers using paper towels. Place blot on the saran wrap. Be careful not to let the blots get too dry.
Step 2. Add ECL Substrate On The Blots
Prepare ECL substrate mixture according to the manufacturer’s recommendations and pipette the substrate mixture on the blot. Place another layer of saran wrap on the blot to create a sandwich. Tap the sandwich in place to prevent it from sliding around when the X-ray film is later loaded into the film cassette (this will result in blurry bands). Remove any excess wash buffer of ECL substrate mix on the sandwich using paper towels or kimwipes.
Step 3. Place X-Ray Film On The Blots In A Film Cassette
Bring the film cassette containing the blots to a dark room. There, you will place a piece of X-ray film into the film cassette by laying it on top of the saran wrap-blot sandwich. Fold a corner of the X-ray film to determine the orientation of the film. Close the film cassette and ensure that the cassette is sealed tightly. Set a timer with the desired exposure time. When detecting new proteins or when new antibodies are used for the first time, remember to start with a one minute exposure time and then increase or decrease based on the intensity of the protein bands obtained. Do not open the film cassette in the presence of light. Only red light is allowed when working with film in a dark room.
Step 4. Remove X-Ray Film From Film Cassette
Once the desired exposure time is reached, carefully remove the X-ray film (in the dark).
Step 5. Place X-Ray Film In The Film Processor
Place it into a film processor machine and keep the lights off until the film is fully developed. Overlay the developed film over the blot and mark the protein ladder with a marker. Other useful information that can be noted on the film are dates, exposure times, antibody concentrations etc. Repeat steps 3-5 if the exposure time has to be modified.
Step 6. Scan The X-Ray Film
The developed X-ray films can be scanned into images using a scanner for use in publications.
