Western blot is a useful technique to detect the presence of and the abundance of proteins in samples. However, it is an unappreciated fact that the effectiveness of western blot in detecting proteins-of-interest depends on the amount of protein samples utilized for the assay. The amount of samples loaded into the SDS-PAGE gel significantly influences the amount of proteins that can be detected in the blots, which will undoubtedly influence the quality of the western blot data and the conclusions that can be obtained from the experiment. In turn, the amount of samples loaded into the gel depends on both sample concentration and volume. Ultimately, the western blot loading amounts have to be sufficient for the detection of proteins-of-interest and uniform across all wells to ensure the data is quantifiable.
A good quality western blot can only be achieved when appropriate concentration and volume of samples are loaded into the SDS-PAGE gel. Choose a known housekeeping protein as a loading control to ensure uniform loading and determine the maximum loading volume based on the gel’s thickness and number of wells.
Factors Affecting Western Blot Loading Amount
The amount of sample to use for a western blot depends on the total protein availability, abundance of proteins-of-interest, sample purity, binding affinity of antibodies, ECL substrates, and the number of target proteins to probe.
Total Available Sample
Experimental samples are often scarce and are available in limited quantities and certain experimental conditions may result in low protein yield. Therefore, low amounts of samples are available for western blotting.
Known Abundance Of Proteins-Of-Interest
If the abundance of protein-of-interest is known to be high, a lower loading amount should be used. A large amount of 100ug of proteins is commonly used by researchers to detect lower abundance proteins (Gosh et al., 2014). However, loading large amounts of proteins may adversely affect binding of low abundance proteins to the membrane. This is because highly expressed proteins will over-saturate the membranes. The best solution to overcome this issue is if the proteins-of-interest are novel and protein abundance is unknown (likely low), a western blot for different protein concentrations (e.g. 1ug, 5ug, 10ug, 20ug) should be done first on a sample to optimize the protein loading amount before utilizing the experimental samples.
Purity Of Samples
A lower amount of purified/semi-purified protein samples is needed to be loaded into the gel as compared to cell lysates (Murphy and Lamb, 2013). This is because purified protein samples typically have a higher concentration of proteins-of-interest.
Binding Affinity Of Primary Antibodies
Primary antibodies with higher binding affinity will detect proteins-of-interest more readily than those with lower affinity. Therefore, if the primary antibodies used to probe for the proteins-of-interest have a high binding affinity, lower protein loading amounts can be used. Often, certain commercial antibodies may recognize non-specific proteins that are not the target protein. These antibodies can still undergo protein-protein conjugation if the non-specific bands are sufficiently far from the target protein band such that there are no overlapping signal bands. Reducing protein loading amount can sometimes decrease the signal from non-specific proteins. This method works if the proteins-of-interest are expressed in moderate to high levels.
Type Of Ecl Substrates
ECL substrates with high sensitivity developed through antibody conjugation methods are able to detect proteins that are in low abundance (Mruk and Cheng, 2011). This is because the substrate requires a lesser amount of HRP to produce chemiluminescent signals. This means that a lesser amount of primary and, subsequently, HRP-conjugated secondary antibodies are needed to be bound to the membrane. Therefore, a lower sample loading amount may be needed for sufficient protein detection.
Number Of Proteins-Of-Interests To Be Probed
A single chemiluminescent-based western blot can be probed for multiple proteins but using a method called ‘stripping’ where the ECL reagents, primary and secondary antibodies can be removed from the blots. The blots can then be re-probed using different primary antibodies and be detected via other chemiluminescence methods such as enzyme-catalyzed bioconjugation. However, ‘stripping’ results in the loss of proteins bound to the membrane (Alegria-Schaffer et al., 2009). Therefore, if there are multiple proteins-of-interest that need to be probed on one single blot, higher protein loading amounts may be required.
What Is A Loading Control In Western Blots?
A loading control uses housekeeping proteins that are highly expressed and abundant to ensure samples have been loaded equally in the western blot so band concentrations can be compared.
Every western blot requires the use of loading controls. Loading controls not only ensure samples have been loaded uniformly in a gel such that protein amounts can be quantified but also can be used to check if the even transfer of proteins from the SDS-PAGE gel to the membrane has occurred. These proteins are also known as housekeeping proteins. Primary antibodies that bind to these proteins will essentially be used as probes for these proteins, thus serving as loading controls. So, what makes a good loading control? Good loading controls are proteins that are constitutively expressed and known abundance with a molecular weight that is different from the proteins-of-interest.
There are numerous commonly-used loading controls (of different sizes) that can be found in different subcellular compartments:
| Molecular Weight (kDa) | Cytosol | Nucleus | Cytoskeleton | Mitochondria | Serum |
| >120 kDa | Vinculin | ||||
| 70-80 kDa | SDHA | Transferrin | |||
| 60-70 kDa | Lamin B1 | Albumin | |||
| 50-60 kDa | Alpha Tubulin | HSP60 | |||
| 40-50 kDa | Beta TubulinBeta Actin | ||||
| 30-40 kDa | GAPDH | TBP/ TFIID | VDAC1 | ||
| 20-30 kDa | PCNA | Cofilin | COX4 | ||
| <20 kDa | CYclophilin A | Histone H3 | Cytochrome C |
The appropriate loading control should be selected based on the nature of the proteins-of-interest. For instance, for detecting cytosolic proteins, cytosol and/or cytoskeleton proteins such as GAPDH and beta Tubulin are suitable loading controls. For detecting proteins in the serum, loading controls such as transferrin or albumin will be suitable.
By having good quality signals from loading controls, relative quantification can be performed to determine the abundance of the protein-of-interest. This can be done by first determining the signal intensity of each band (protein-of-interest and loading control), then normalizing the intensity of the protein of interest to the loading control (Novus Biologicals). This provides information on the abundance of the protein-of-interest relative to the housekeeping protein.
If you are looking for great western blot results and want to learn more best practices, read our article on how to choose the best western blot negative control.
How To Measure Western Blot Sample Protein Concentrations
To determine western blot loading amounts to deliver into the gel to achieve desired volumes, the concentrations of protein samples have to be measured and quantified by using UV absorbance, the Bradford assay, and/or the BCA assay.
There are three main methods to determine protein concentrations in samples:
Method 1. Uv Absorbance
Protein concentrations can be assessed by measuring the UV absorbance at 280 nm. At 280 nm wavelength, a strong signal peak can be detected from proteins due to the absorbance from amino acid residues such as tryptophan. If the proteins-of-interest do not contain absorbable residues, tryptophan bioconjugation methods can be performed to generate signals. The peak signal can then be converted into protein concentration using the Beer-Lambert law (Clark, 2022). However, this method only provides an estimation as to the protein concentration present in the samples.
Method 2. Bradford Assay
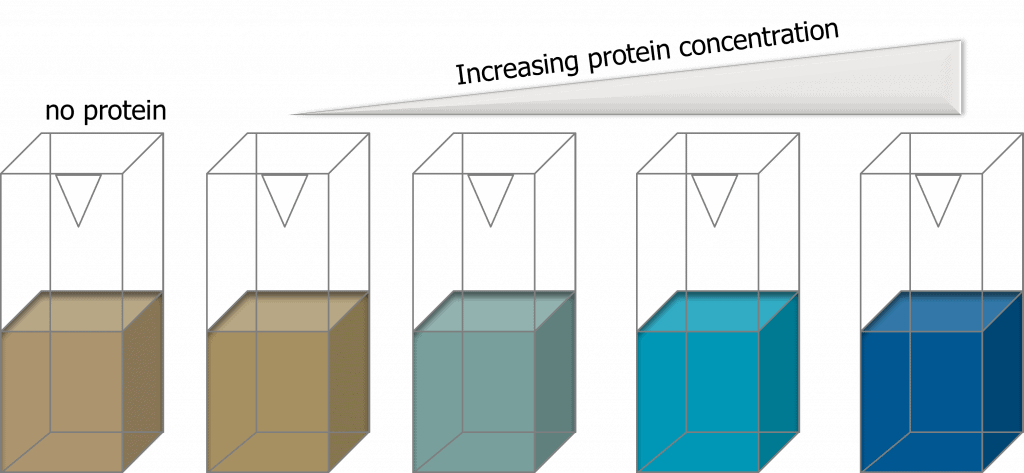
The Bradford assay is a colorimetric-based method that relies on the use of a protein-binding dye called the Coomassie dye (Kielkopf et al., 2020). In acidic conditions, proteins bind readily to Coomassie dye, resulting in a colorimetric shift from a reddish-brown color to a blue color. This is due to the formation of protein-dye complexes. The shade of blue correlates with the concentration of protein presence i.e. the darker the shade of blue, the higher the protein concentration. The reddish-brown color and the blue color have different absorbance maximums – 465 and 610nm respectively and the optimal wavelength to measure the blue coloration is at 595 nm.
Bradford assays are relatively low-cost and straight-forward, making it an attractive method to quantify protein concentrations. However, there is a major disadvantage of using the Bradford assay. Coomassie dyes are incompatible with surfactants that are typically used to solubilize membrane proteins. In the presence of even a trace amount of surfactant, the Bradford reagent will precipitate, rendering it unusable for protein quantification. However, today, there are detergent-compatible Bradford assays that can be used to overcome this limitation.
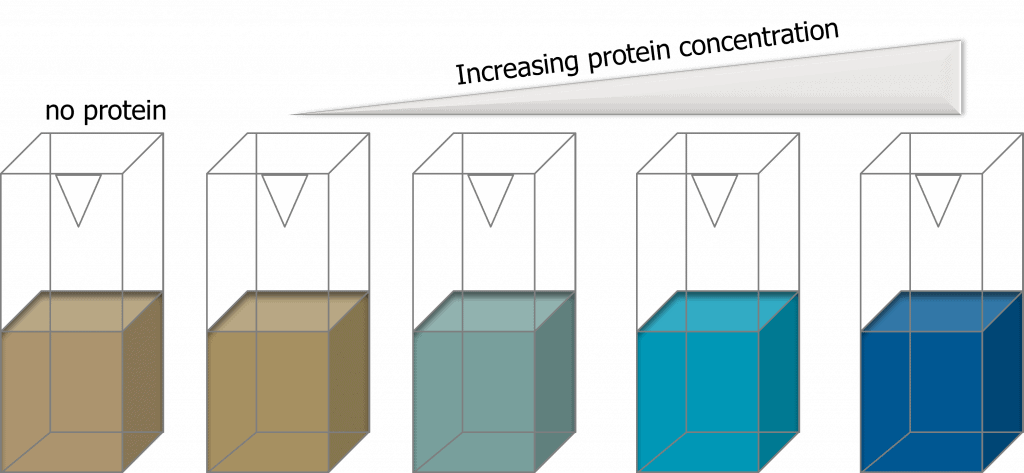
Method 3. Bca Assay
The bicinchoninic acid (BCA) assay is also a colorimetric-based assay that relies on the reduction of Cu2+ ions by proteins, which then bind to BCA to form an intensely-colored BCA-copper complex that has a strong absorbance at 562 nm. An advantage BCA assays have over Bradford assays is that BCA assays are compatible with samples that contain up to 5% surfactant. Another advantage of BCA assays is that there is low protein-protein variation. Different compositions and structures of proteins may result in variation in colorimetric response but BCA assays are less sensitive to protein-protein variation and are thereby more accurate in determining protein concentrations.
Western Blot Maximum Loading Volume
The maximum western blot loading amount for western blots depends on the gel thickness and number of wells in the SDS-PAGE gel.
For example, a 10-well gel (1.0mm thickness) loading volume ranges from 1 to 44 µl. If pre-cast gels are used, please refer to the manufacturer’s instructions as the maximum well volume differs between brands.
Maximum Loading Volumes For Different Standard Gels (ThermoFisher Scientific):
| Number of wells | 1.0 mm thickness | 1.5 mm thickness |
| 5 | 105ul | 160ul |
| 9 | 44ul | 66ul |
| 10 | 44ul | 66ul |
| 15 | 26ul | 40ul |
Western Blot Loading Tips
For best practices to obtain reliable western blot bands, use longer tips to load samples into the gel, add bromophenol blue as a visual aid, avoid air bubbles when loading, use a protein ladder, and add equal volume of blank buffer to empty wells.
Tip 1. Use Gel Loading Tips
Gel loading tips have longer and slender ends which allow the tips to be inserted deeper into the wells to facilitate easy and accurate loading of samples into the wells of the SDS-PAGE gels. This will prevent the flow of samples out of the wells into the running buffer and into neighboring wells.
Tip 2. Add Bromophenol Blue To The Stacking Gel Mixture
Wells of SDS-PAGE gels are invisible, causing sample loading to be difficult. By adding bromophenol blue dye to the stacking gel mixture, the stacking portion of the SDS-PAGE gel will be stained blue, allowing easier sample loading into the wells.
Tip 3. Add Samples Slowly Into The Wells To Avoid Air Bubbles To Form
When samples are dispensed too rapidly into the wells of the SDS-PAGE gels, air bubbles will form and float upwards, which may result in the pushing of some protein samples out of the wells into the neighboring.
Tip 4. Add Protein Ladder To The First Well Of The Gel
Protein ladders/ markers allow for the estimation of the molecular weight of proteins and are also used to track the electrophoretic separation of protein samples (MilliporeSigma).
Tip 5. In Empty Wells, Add A ‘blank Buffer’
In empty wells, load an equal volume of ‘ blank buffer’ to prevent lateral band spreading/ distorted bands (PhosphoSolutions).
