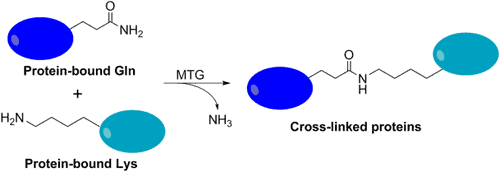
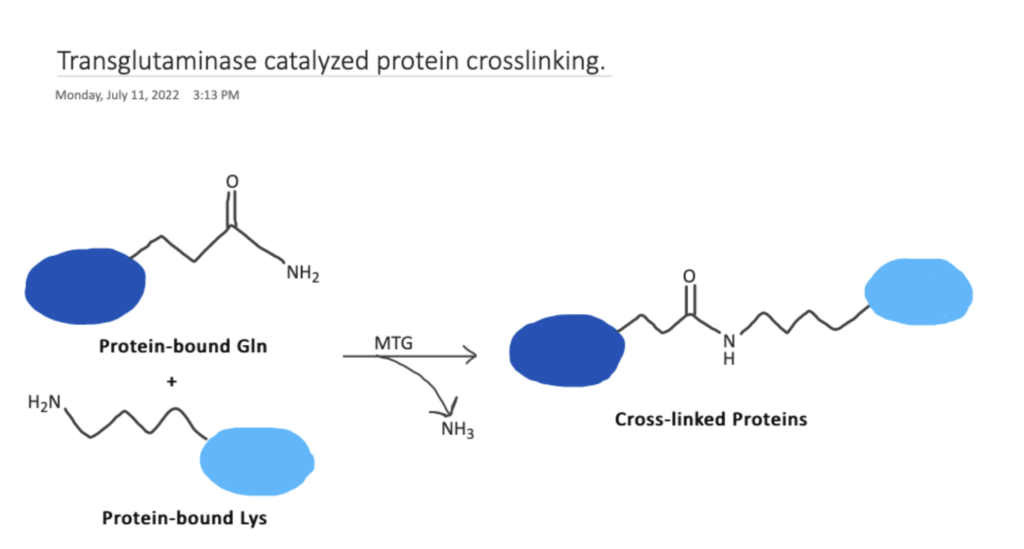
Transglutaminases naturally bring about the development of amide bonds between glutamine side chains and primary amines to form insoluble protein masses. In this article we will discuss transglutaminase bioconjugation chemistry, protocols, and applications.
Transglutaminase bioconjugation chemistry utilizes the acyl transfer reaction between the γ-carboxamide groups of glutamine and the ε-amino groups of lysine resulting in a stable peptide bond. Using this functionality, many molecular structures can be conjugated onto proteins for applications such as, transglutaminase-catalyzed bioconjugation of peptides to proteins, transglutaminase-mediated bioconjugation to antibodies, and q-tag transglutaminase conjugation of radiolabels onto antibodies .
Transglutaminase Bioconjugation Chemistry Overview
The naturally occurring enzyme transglutaminase, enables the formation of amide bonds between glutamine side chains and primary amines, to form insoluble protein masses. Transglutaminase catalyses the acyl transfer reaction between γ-carboxamide groups of glutamine and the ε-amino groups of lysine resulting in a stable peptide bond (source).
Transglutaminase-Catalyzed Bioconjugation of Peptides to Proteins
Transglutaminases can catalyse bioconjugation of peptides to proteins and provide a way to generate photoremovable protein conjugates that are site specific. Using transglutaminase bioconjugation chemistry, it’s possible to control and further study these conjugates. This process, known as protein photoswitching, has become very important in the field of biotic and abiotic processes. Since there are only a few ways to apply these switches into proteins in specific areas, this novel site-specific technique of creating photoremovable protein conjugates is noteworthy (source). You can find a more detailed protocol in This article from MDPI . Besides transglutaminase-catalyzed bioconjugation, you might consider arginine bioconjugation or tyrosine bioconjugation as strategies for site-specific labeling as well.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
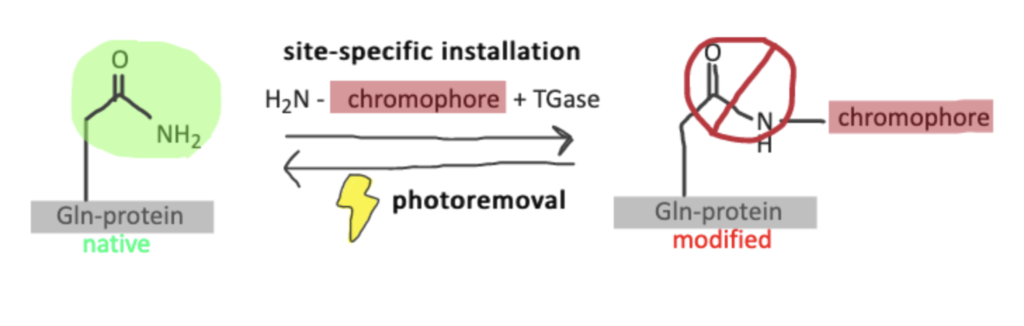
Step 1. Transamidation
Amine-containing chromophores (e.g., containing photocleavable linkers such as o-nitrobenzyl and o-nitrophenylethyl groups) are incorporated via transamidation into a glutamine side chain of α-gliadin, LCMV, and TAT peptides.
For more information regarding photocleavable linkers, read our article on bioconjugation linkers.
Step 2. Transglutaminase Catalysis
β-casein and UmuD proteins are introduced by transglutaminase (TGase, EC 2.3.2.13) conjugation.
Once conjugated, it’s possible to utilize the photocleavable groups on the chromophores (such as o-nitrobenzenes) for photolysis. The photolysis regenerates the original peptides and proteins. If this modification causes certain activities to slow down or stop, it is called caging. This technique is important because it is uncomplicated, robust, and can easily be adapted, as all the elements are widely obtainable.
Transglutaminase-Mediated Bioconjugation to Antibodies
Transglutaminase-mediated bioconjugation is an effective method for developing antibody conjugates. In nature, transglutaminase catalyzes amide bond formation between glutamine side chains and amines. This activity can be utilized for making site-specific conjugates. You can find a more detailed protocol in this article.
Below we provide a general protocol for using transglutaminase for site-specific bioconjugation to antibodies using transglutaminase bioconjugation chemistry.
Related articles:
- You might also consider electrochemical bioconjugation to tryptophan, for example, as an alternative method for site-selective bioconjugation. We’ve written about this in our other article.
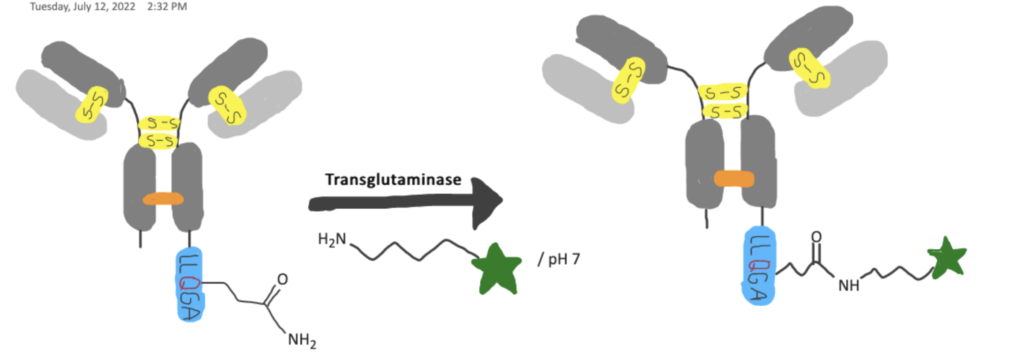
Step 1. Introduction of Specific Peptide Recognition Sequences
Specific peptide recognition sequences are introduced into the monoclonal antibody via saturation mutagenesis due to the lack of original sites for transamidation. The recognition motif can be obtained from Streptomyces papain inhibitor (SPIP ), Transglutaminase’s original substrate.
These sequences produce better chemical kinetics when tested in model peptides and in biotinylation of Her2-targeting antibody trastuzumab variants. Also, antibody-drug conjugates collected from trastuzumab, which has been C-terminally tagged with the novel recognition sequence, produces an improved payload-antibody ratio.
Step 2. Rapid and efficient mTG-catalyzed Bioconjugation
Incubation with an amine containing compound and the antibody leads to a rapid and efficient mTG-catalyzed bioconjugation.
Q-Tag Transglutaminase Conjugation of Radiolabels onto Antibodies
Being able to label antibodies has made a huge difference in the development of clinical imaging tools. Being able to apply site- and regio-selective therapeutic approaches enables manufacturers to develop consistent batches which have predictable therapeutic profiles and activity. Heterogeneity typically leads to less predictability (source). To learn about alternative methods radiolabeling onto antibodies, consider our article about bifunctional chelating agents and how we use them in conjugation.
Despite previously suggested high regioselectivity of TGases, in the anti-Her2 Ab (Herceptin™), mass spectrometry indicates only 70–80% functionalization at the target site (Q298H), in competition with modification at other sites, such as Q3H critically close to the CDR1 region. This means that Transglutaminase (TGase) is able to catalyze chemical reactions of glutamine (Q) residues in a sequence of recognition (the ‘Q-tag’) and bypass other glutamines in heavy chains of IgGs. This paper gives a very detailed outline of this transglutaminase bioconjugation method.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
Step 1. Creation of Protein Substrate
Create the protein substrate, deglycosylated (dg) Herceptin (dg-Her), by treating wild-type Herceptin (wt-Her) with PNGase.
Step 2. Conjugate Protein to Azidoamine
dg-Her is then conjugated to the azidoamine H2N–CH2CH2–(OCH2CH2)2–N3 using the TGase from Streptomyces mobaraensis to introduce an azide residue into the side chain of Q298H (creating azido-Her) to react with alkynes that have been strained.
Step 3. Click Drugs onto Azido-Herceptin
Using drugs that have been conjugated to strained alkynes, it’s now possible to click onto the Herceptin in a side-specific manner.
Other Applications of Transglutaminase-Based Bioconjugation Chemistry
Transglutaminases are responsible for cross-linked, generally insoluble protein polymers, that are integral for forming boundaries, barriers and stable formations. Some of these processes include blood coagulation as well as the formation of hair and skin. Transglutaminase catalysed processes are considered to be irreversible, and should be managed and observed using controlled procedures.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Application 1. Microbial Transglutaminase and its Application in the Food Industry
The enzyme transglutaminase that naturally catalyses the formation of protein isopeptide — ie. Transglutaminase bioconjugation chemistry is used for protein-protein bonding. This cross-linking characteristic is used extensively in different processes: in the manufacturing of dairy products such as cheese, in the processing of meat products, for the production of coatings and films that are suitable for human consumption, and bakery product manufacturing.
Transglutaminase has an amazing ability to enhance the density, consistency, elasticity and water-binding ability of products in the food industry. In 1989, Streptoverticillium was used to produce microbial transglutaminase. Transglutaminase processes are used widely throughout most fields of industry because of their wide selection and the lower costs of these biotechnological procedures. This paper explains more about features and applications of transglutaminase. Another key benefit of using transglutaminase is that it catalyzes bioconjugation in water unlike some techniques which require organic solvents.
Application 2. Applications of Transglutaminase in Wool, Silk and Leather Processing
Wool
Wool is a natural fiber produced by skin cells of sheep, goats, rabbits and other animals. It is heavily processed before it can be used for the production of clothes. These processes include scouring, carding, gilling, combing, drafting, spinning and twisting. Various chemicals and enzymes are used in the treatment and change the properties in the wool in a negative way.
Transglutaminase is used to repair the damage caused by the treatments at the various stages. The enzyme catalyzes protein-protein conjugation and improves the overall strength, prevents shrinking, improves softness, improves wettability for optimal dyeing, improves flexibility, and aids in protection from microorganisms.
Silk
Transglutaminase-based processing is very effective in developing characteristics that make silk fabric more crease resistant. It also repairs the damage caused by pretreatments and reduces wrinkling of silk fibers. Treatment of silk with mTGase followed by ultrasound yielded better results than other treatments, bringing about a 17.4% improvement in wrinkle recovery and a 11.2% improvement in strength.
As with wool fibers, hydrogen peroxide and protease treatment can damage the structure of silk fiber. Pretreating the fibers opens up the outer structure of the strands allowing the mTGase to enter the fiber. It was found in another investigation, that thermally aged silk could be repaired and strengthened by using transglutaminase-mediated polymerization.
Leather
Filling in the processing of leather is exactly as the word implies – it refers to “filling” of spaces and voids in the leather fibers using suitable materials. This is an important step to smoothen out the leather and remove the lines and irregularities on the surface of the leather. Filling materials often remain in place during the later processing steps.
The filling process is done using products such as glucose, flour and gum. Along with these gelatin and casein are utilized as effective filling solutions. Various substances such as glutaraldehyde combined with gelatins have resulted in highly polymerized gelatins to fill leather effectively.
Treatment of casein with transglutaminase has produced similar materials that feel like leather. This means that it is possible that, with mTGase treatment, affordable proteinaceous commercial products can be created via Transglutaminase treatment instead of expensive filling materials currently used. (source)
