When designing a conjugated molecule, you might need to use bifunctional crosslinkers to get your desired structure. Bifunctional crosslinkers, or crosslinking agents, are molecules that contain two or more reactive “ends” that allow you to chemically bond two desired molecules together. There are a variety of bifunctional crosslinkers available on the market with many options for functional groups and reaction conditions. Because of this variety, it can be difficult to decide which is the best crosslinking agent to use. In this article, we explore the different types of bifunctional crosslinkers, why and when to use a crosslinker, give you some example applications, and, finally, explain how to choose the right bifunctional crosslinker for conjugation reactions.
To choose the right bifunctional crosslinkers, first assess functional groups on molecules that need to be conjugated. Then select complementary functional groups on the crosslinker and determine whether the reaction can be orthogonal. Ensure that there are other bifunctional crosslinkers that can be utilized for the conjugation reaction, as a backup.
What is a Bifunctional Crosslinker or Crosslinking Agent?
A bifunctional crosslinker, also known as a crosslinking agent, is a type of chemical reagent that has two or more functional groups at its “ends”. These reagents can be used to literally cross-link two molecules together using suitable functional group reactions. Bifunctional crosslinkers are most commonly used in polymer synthesis reactions and in conjugation chemistry, particularly bioconjugation reactions.
A bifunctional crosslinker, or crosslinking agent, is a molecule with two or more functional groups that are used to cross-link two or more molecules together. Types of bifunctional crosslinkers include homobifunctional crosslinkers, heterobifunctional crosslinkers, and photoreactive crosslinkers.
There are several different types of crosslinkers used in conjugation reactions. Here are the most common types:
Homobifunctional Crosslinkers
Homobifunctional crosslinkers are crosslinking agents that have the same functional chemistry at both ends of the structure. This comes with the added advantage of only needing to perform a one-step chemical crosslinking reaction in most cases. The most common homobifunctional crosslinkers are simple saturated carbon chains with two identical functional groups on each end, such as a diol or diamine.
Heterobifunctional Crosslinkers
Heterobifunctional crosslinkers are crosslinking agents that have different functional groups at each end of the structure. This comes with the main advantage of more control over the synthesis through multi-step reactions. An example of a simple heterobifunctional crosslinker would be a carbon chain with a thiol on one end and an amine on the other.
Photoreactive Crosslinkers
Photoreactive crosslinkers have more complicated chemistry than the previously described types of crosslinker. These crosslinkers have photoactivatable functional groups that only become reactive when exposed to UV or visible light. These molecules typically use less common functional groups and more niche chemistry. Their main advantage is that you have control over when the reaction starts. For example, you could add the photoreactive crosslinker to one of your molecules, make further modifications, then activate the photoreactive group for the conjugation step. The most common functional group used in photoreactive crosslinkers is the aryl azide group which activates in UV light.
Reasons to Use a Bifunctional Crosslinker
When you’re planning a conjugation reaction, there are many good reasons to use a bifunctional crosslinker.
Reasons to use a bifunctional crosslinker include the ability to control the size of conjugates, increased conjugate stability and improved detection and ability to analyze conjugates once they are created.
Allows You To Control The Size Of Your Conjugate
One of the most important reasons for using a crosslinker is because it allows you to control the distance between the two molecules you’re conjugating. This can allow you to avoid things like steric hindrance and other unfavorable interactions between your two molecules. It can also allow you to optimize other properties of your conjugate like fluorescence or enzymatic activity. For example, in this study, the authors showed that the length of the crosslinking “spacer” affected the drug release and therapeutic efficacy of their antibody-drug conjugate against tumors.
Alternatively, if you need your molecules as close together as possible, you could consider using a zero-length crosslinker, which is the smallest available reagent you can use to form a bond between your two molecules. Enzymatic bioconjugation techniques are a great way to introduce site-specific zero-length attachments.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Increased Conjugate Stability
By choosing the right bifunctional crosslinker, you can effectively improve the stability of your conjugate molecule. This increased stability can be provided by steric or electronic protection of the conjugate’s bonds during further reactions, increased resistance to hydrolysis in storage, or even thermal stability. For example, in this paper, the authors significantly increased the stability of their gold nanoparticles (AuNPs) by adding a photo-crosslinked polystyrene shell. This particularly improved their bioconjugation with streptavidin using noncovalent and covalent methods. We’ve discussed bioconjugation of nanoparticles in more detail in another article.
Improved Detection And Analysis
The use of larger and more specific bifunctional crosslinker molecules can give you better detection and analysis by providing a distinct structural market to confirm the formation of your target conjugate. In situations where you need the bond to be cleavable, it can also help you to more easily detect whether the bond was broken correctly. For example, in this paper, the authors created a pyrene-maleimide based fluorescent cross-linking reagent that they were able to use to derive information about the proximity of functional groups in protein. Maleimides are excellent coupling functional groups to attach cysteines on proteins. Palladium bioconjugation is an alternative site-specific method to attack thiols on cysteines.
When to Use a Heterobifunctional Crosslinker
Heterobifunctional crosslinkers offer several advantages over their homobifunctional alternatives.
Use a heterobifunctional crosslinker for increased precision while conjugation, conjugation of two different functional groups, and to control the conjugation with two functional groups within one-pot.
- You want more precision. Using a heterobifunctional crosslinker is more precise because it allows you to control the progression of the synthesis in at least two steps. This means you can get better reaction yields and fewer byproducts than you would get if you used a homobifunctional reagent. It also means you can add protecting groups and other modifications between the two conjugation steps.
- You’re conjugating two very different molecules. If your two target molecules are very different in terms of structure and functional groups, it will be difficult to find a homobifunctional reagent that lets you create your desired conjugate. By having two or more functional groups, you have more control over your synthesis, allowing you to add your crosslinker to your first molecule with one reaction, and then add the next molecule using different chemistry.
- You have more than two starting molecules. If you’re creating a structurally complex conjugate that has multiple molecules cross-linked together, it will be significantly easier to use a heterobifunctional crosslinker with multiple functional groups. These groups can be protected or manipulated, allowing you to have a high degree of control over your synthesis.
How to Choose a Bifunctional Crosslinker
Here’s how to choose a bifunctional crosslinker for your next conjugation reaction.
To choose a bifunctional crosslinker, first determine which functional groups to use for reactions on both molecules that are to be conjugated. Then identify complimentary functional groups and find a bifunctional crosslinker that includes both functional groups.
1. Determine Which Functional Groups To React on Your Molecules
Depending on what type of conjugate you’re trying to make, you’ll need to determine the available functional groups on your target molecules. For example, if you’re performing bioconjugation reactions using things like proteins, you’ll typically be working with common biological functional groups like amines, carboxylates, etc. However, if you’re conjugating inorganic compounds, like polymer nanoparticle compounds, you’ll have more flexibility to use diverse functional groups not commonly found in nature like alkynes, halogens, and even metals. We’ve covered bioconjugation to metal complexes in this linked article.
2. Choose Complimentary Functional Groups on the Crosslinker
The next step is to choose complementary functional groups on your crosslinker so that you can create the covalent bonds for your conjugate. For example, if you’re using carboxyl groups on a protein as your target, you may choose an amine as your complementary functional group. This gives you access to various different types of crosslinkers and reactions like carbodiimide chemistry (using EDC). Similarly, if you’ve got a thiol group on your biological molecule you may use maleimide chemistry, an example of which can be found above. Alternatively, if you’re planning to use a photoreactive crosslinker step in your synthesis, you might consider alkyne/azide as your complementary functional groups.
3. Determine Whether Reactions for the Functional Groups Can Be Orthogonal
Once you’ve chosen appropriately complementary functional groups, it’s important to determine whether the reaction can be orthogonal. This means that you can perform your reaction steps in a “one-pot” or “click” style (like with azide bioconjugation techniques) where the reactions can be performed simultaneously and selectively. This requires significant synthetic planning and optimization, but is achievable with the right functional groups and conditions. For example, the authors in this work explored orthogonal reactions for protein-DNA conjugation for force spectroscopy.
Using orthogonal conjugation reactions is a great way to avoid common bioconjugation problems like a lack of site-selectivity.
Polymers can be conjugated to proteins and antibodies using a range of functional groups such as amines, carboxyls, or thiols. Explore conjugation kits for polymers, proteins, and antibodies, here.
4. Purchase Backup Crosslinkers That Utilize Other Chemistries
It’s always a good idea to purchase backup crosslinkers that utilize other chemistries so you have an alternative should your initial synthesis fail. Many major chemical and biochemical suppliers, such as Thermo Fisher, offer a variety of crosslinkers that might suit your needs. Alternatively, you may need to explore niche crosslinker suppliers who produce crosslinkers with uncommon functional groups and structures. Here are some examples of common biomolecules with their functional groups and complimentary crosslinker groups:
| Biomolecule | Common Functional Group | Complimentary Functional Group on a Crosslinker |
| Protein | Carboxylate | Amine |
| Protein | Amine | NHS-Ester |
| Protein | Thiol | Maleimide |
| DNA | Nucleotide amine | NHS/EDC |
| DNA | Organic phosphate | EDC/imidazole |
| Carbohydrate | Hydroxyl | Isocyanate |
| Carbohydrate | Aldehydes | Hydrazine |
Applications of Bifunctional Crosslinkers for Conjugation Reactions
Bifunctional crosslinkers have been used in a variety of applications in conjugation reactions.
Applications of bifunctional crosslinkers for conjugation reactions include protein-protein conjugation, polymer-gold nanoparticle conjugation, and antibody-drug conjugation.
Protein-Protein Conjugation
Crosslinking reagents are extensively used in protein-protein conjugation chemistry. The effectiveness of the crosslinking largely depends on the availability of specific functional groups on the protein surface. The most common targets for protein-protein conjugation are amines, carboxyls, sulfhydryls, and carbonyls, all of which are commonly found on proteins in varying quantities. For more information on protein-protein conjugation techniques, read our other article.
One commonly used crosslinker for protein-protein conjugation is SMCC which uses NHS ester chemistry on one end and maleimide thiol chemistry on the other. Sadly, this reaction isn’t very stable and results in a large amount of unwanted crosslinked by-products.
Here’s the basic scheme for that reaction:
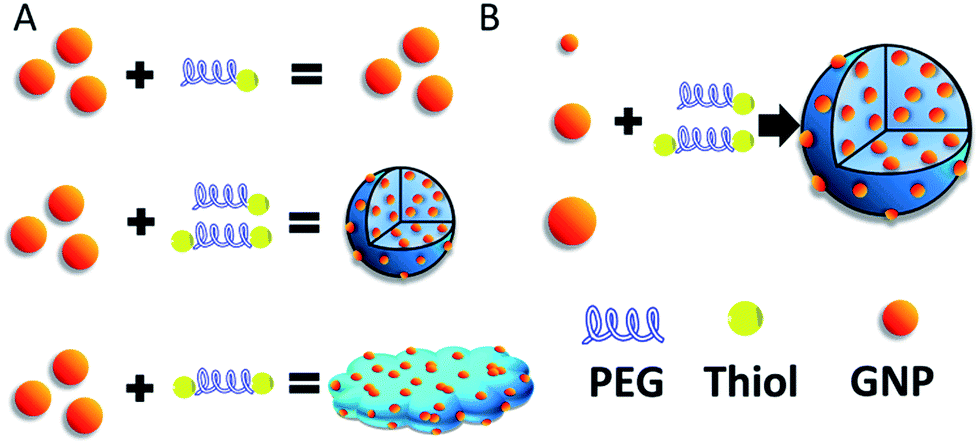
Polymer-Gold Nanoparticle Conjugation
As mentioned in the example above, crosslinkers can be used during polymer-gold nanoparticle conjugation. Gold nanoparticles are already of high interest for their chemical and biological applications. The conjugation of various different polymers to a gold nanoparticle, such as polystyrenes, polyethylene glycol (PEG), and more, can enhance its properties in various applications. For example, the authors of this work used a dithiol-PEG structure to create inter nanoparticle cross-links that could be used for drug delivery. These were then tested against cancer cells because of their enhanced uptake and subsequent decomposition in the tumour microenvironment.
We’ve covered more information on pegylation and peg bioconjugation techniques in another article.
Antibody-Drug Conjugation
Another popular use for crosslinkers for conjugation reactions is to create antibody-drug conjugates which can have enhanced drug delivery and stability in the body. While there are many examples of this in the literature, one of the most interesting recent studies used sequence-defined cross-linkers to alter the hydrophobicity of their antibody-drug conjugate and improve its in vitro potency. The authors used a sequence-defined PEGylated cross-linker which, when attached distally from the payload of monomethyl auristatin E, had significantly improved properties.
Structure of the sequence-defined antibody-drug conjugates (ADCs) (source).
