β-Amino alcohols are versatile intermediates in the preparation of a wide variety of biologically significant compounds. The synthesis of these structures can be achieved by various routes, with the most straightforward and high-yielding way involving heating epoxide in aprotic solvents with excess amines. However, this classical method presents a number of limitations, such as slow reaction rates due to the low nucleophilicity of amines, the sensitivity of epoxides, the requirement of excess inorganic bases, and the reduction in regioselectivity. As such, researchers have developed a variety of methods and materials/catalysts that can enhance the electrophilic character of epoxides and the effectiveness of bioconjugation using epoxides, as discussed below.
Bioconjugation using epoxides typically involves a ring-opening reaction catalyzed by Lewis acids, nanometals, and irradiation techniques.
The Reaction Of Epoxides With Amines
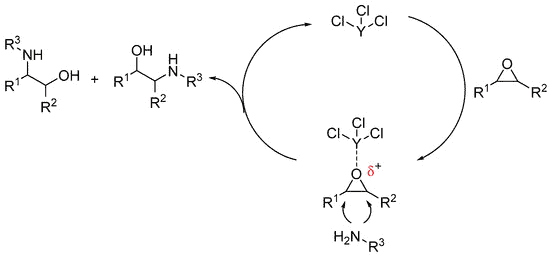
Epoxides can react with amines by either SN1 or SN2 mechanisms to yield β-Amino alcohols.
Epoxides are a class of valuable carbon electrophiles found both in nature and in modern organic synthesis. With 27 kcal/mol of ring strain, these three-membered heterocycles are highly reactive with various nucleophiles in a non-enzymatic ring-opening process. The reaction can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and the reaction conditions (Moschona et al., 2020; Bertolini 2002).
Under acidic reaction conditions, the ring-opening occurs via the SN1 mechanism involving two key steps. First, the oxygen of the epoxide is protonated by the acid to make it more electrophilic and to create a better leaving group. Then, the best nucleophile present in the solution (water, alcohol, etc.) performs a ‘backside attack’ at the carbon of the epoxide at the ‘more substituted’ position. Final deprotonation of the nucleophile then gives the neutral product, and the two leaving groups—the nucleophile and OH—are oriented trans to one another (Huang et al., 2022; Thirumalaikumar, 2002).
Highly reactive nucleophiles react with epoxides under basic conditions via an SN2 mechanism. As there is no acid available to protonate the oxygen, the ring is unlikely to open without a ‘push’ from the nucleophile. The ‘less substituted’ carbon of the epoxide is the site of the nucleophilic attack and the leaving group is the oxygen atom of the epoxide in the form of the alkoxide anion—a poor leaving group—that is is then converted to the alcohol on an acidic workup. More on the ring-opening reaction of epoxides can be found in this review.
Protocol For Bioconjugation Using Epoxides
Protocols for epoxide bioconjugation typically utilize Lewis-acid catalysis, microwave-assisted catalysis, or nanometal catalysis.
Method 1. Metal Triflate-Catalyzed Epoxide Bioconjugation Reaction
Lewis acid catalysis of epoxides has been employed in complex molecule synthesis for decades. Lewis acid activates epoxides by facilitating C–O bond polarization and priming them for the nucleophilic attack, producing epoxides with flexible reactivity (Hansen et al., 2021; Wilson & Denmark, 2016). Recently, metal triflates such as rare-earth metals have received increasing attention for their role as ‘Lewis superacids’ in a number of reactions (Kobayashi, et al., 2002; Natongchai et al, 2017).
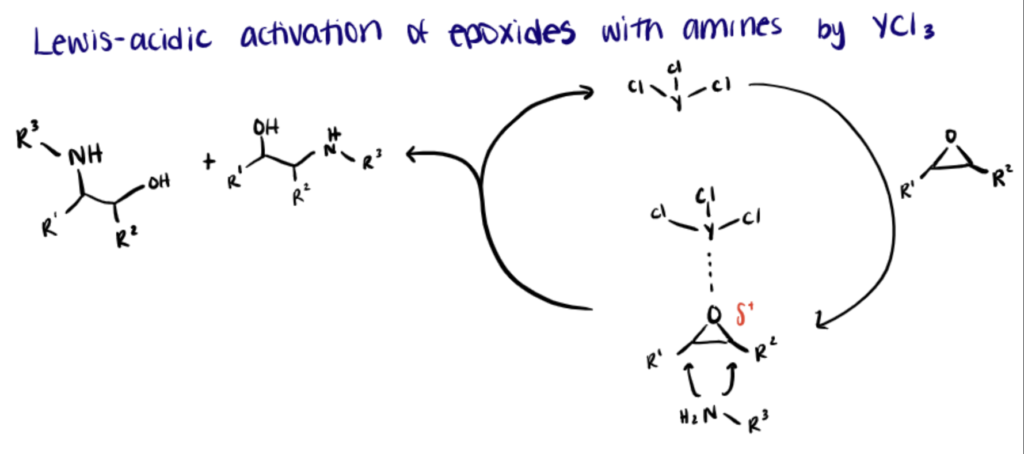
In the following section, we briefly describe the YCl3-catalyzed ring-opening reaction of epoxides with amines under solvent‐free conditions utilized by Natongchai et al., (2017). For more details, refer to this paper.
Step 1. Stir Epoxides with Amines
First, the authors stirred a mixture of epoxide and amine at room temperature in the presence of YCl3. After the reaction (3 h, room temperature, solvent-free), the crude mixture was purified by column chromatography using silica gel as stationary phase.
Step 2. Activate the Epoxide
YCl3 activates the epoxide ring through interaction with the oxygen atom of the epoxide, making the epoxide ring more vulnerable to nucleophilic attack by amines. The nucleophilic attack reaction occurred at the less hindered side of the epoxide ring (SN2 mechanism) and was found to be regioselective for most of the substrates.
Step 3. Characterize Synthesized Products with Spectroscopy
All products were identified by spectroscopic studies and the results were compared with the previously reported catalysts data used for the synthesis of β-amino alcohols. The results indicated that the loading of YCl3 gave a 100% conversion yield with excellent regioselectivity, demonstrating a highly efficient catalytic strategy for the synthesis of β-amino alcohols.
Method 2. Microwave-Assisted Epoxide Bioconjugation Reaction
Compared to hydrolysis-mediated ring-opening for maleimide bioconjugation, the microwave-assisted ring-opening reaction of epoxides has been considered as a convenient, green, and highly efficient regioselective approach. In contrast to the conventional heating process—in which heat transfer occurs via convection—microwave irradiation offers shorter reaction times, expanded reaction range, and fewer by-products (Nadargi et al., 2020; Kumar et al., 2020).
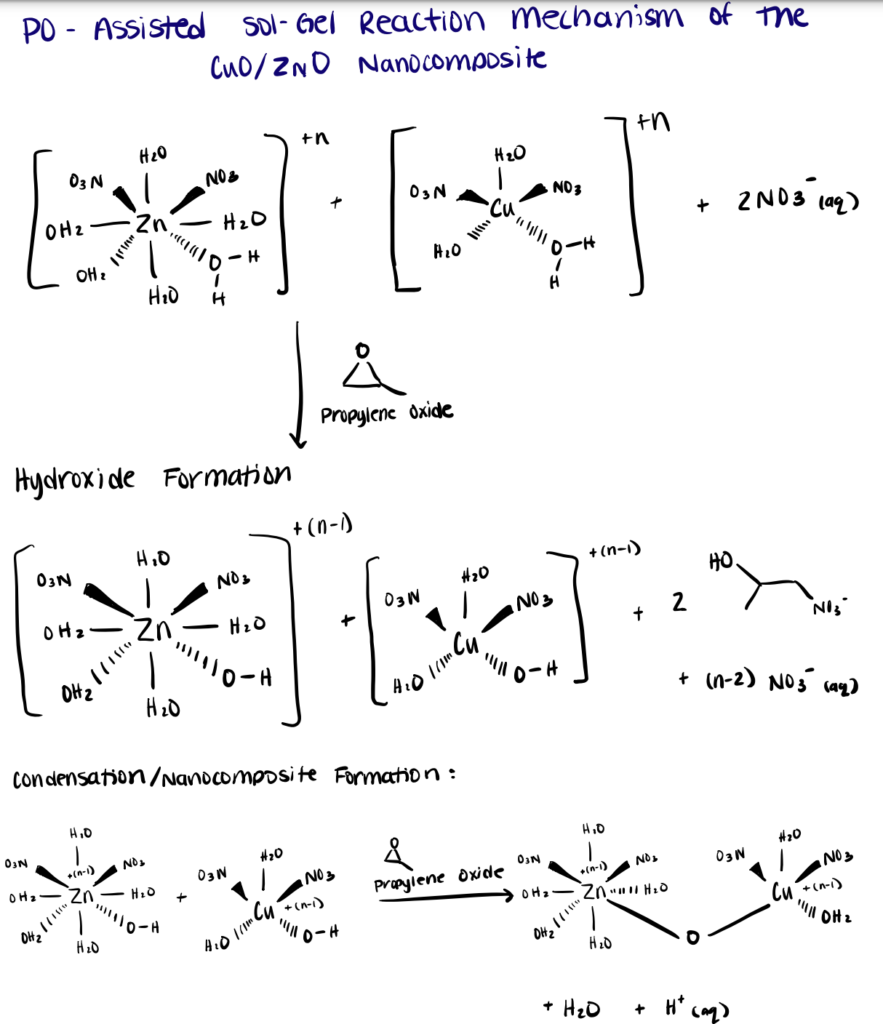
In the following section, we briefly describe a robust synthesis approach of CuO/ZnO nanocomposites using microwave-epoxide-assisted hydrothermal synthesis utilized by Nadargi et al. (2020). For more details, refer to this paper.
Step 1. Stir Epoxides with Metal Salts
First, a mixture of zinc nitrate hexahydrate (Zn(NO3)2·6H2O), copper nitrate trihydrate (Cu(NO3)2·3H2O) in aqueous media with propylene oxide as the proton scavenger/gelation agent was prepared. During the reaction (15 min, constant stirring), the epoxide ring was attacked by the nucleophile, namely the free uncoordinated corresponding anion of the metal salt (NO3–).
As the ring opens, a proton from a water ligand molecule is abstracted, thereby yielding the mixture of nitrate-aqua metal hydroxides. The formation of metal-oxygen–metal bonds observed during the condensation reaction was viewed as a ligand-substitution reaction. As water is an easily detachable group, the kinetics of the reaction is primarily determined by the pH and nature of the attacking nucleophile. See our article on bioconjugation in water for tips on reactions in aqueous solutions.
Step 2. Synthesize Nanocomposites Using Microwave-Assisted Hydrothermal Process
The obtained mixed metal hydroxides solids were treated to microwave hydrothermal process (1400/900 W, 40% power, 10 min) to yield the high-surface-area CuO/ZnO nanocomposite.
Step 3. Characterize Cuo/Zno Nanocomposites with Elemental Mapping
Elemental mapping displayed the good dispersion of Cu in the ZnO nanocomposite matrix. Additionally, the materials demonstrated high sensitivity and selectivity toward H2S gas in comparison with the reducing gasses and volatile organic compounds.
Method 3. Nanometal-Catalyzed Epoxide Bioconjugation Reaction
A variety of metal-catalyzed processes have served as the backbone of the conventional strategies for the activation of epoxides to synthesize asymmetric β-amino alcohols, as well as the bioconjugation of metal complexes. Among them, strategies utilizing nanoscale metal-organic frameworks (MOFs) have found enormous applications for optimizing the selective catalytic performance of epoxidation reactions. MOFs serve as a class of highly tunable hybrid materials with remarkable properties and design versatility due to their great surface area, high stability, open channels, and permanent porosity (Beyzavi et al., 2015).
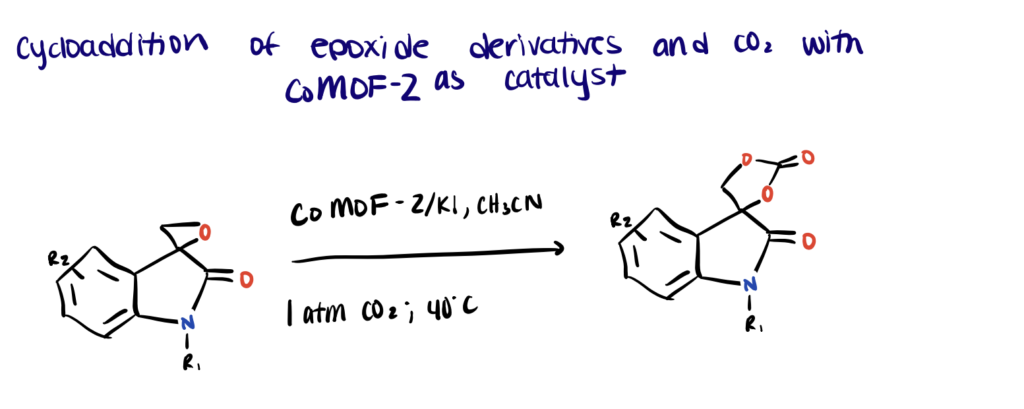
Here we describe a well-defined and highly selective reaction for the cycloaddition of CO2 to epoxides using CoMOF-2 catalyst by Parmar et al. (2019). For more details, refer to this paper.
Step 1. Synthesize the CoMOF-2 Catalyst and Oxindole Epoxide
First, the microporous mixed ligand CoMOF-2 catalyst possessing functionally decorated amide groups was synthesized. The following physicochemical characterization of the CoMOF-2 showed good thermal/chemical stability, accessible porosity, densely populated metal sites, and strong CO2 adsorption properties. The oxindole-based epoxides were synthesized and characterized according to this protocol.
Step 2. React Oxindole Epoxides with CO2 Using CoMOF-2 Catalyst
Cycloaddition of oxindole epoxide substrates and CO2 was carried out in the presence of CoMOF-2 (RT or 40 °C, 600 rpm). Co-catalysts were added wherever applicable to optimize the reaction. Upon completion of the reaction, the catalyst CoMOF-2 was recovered and can be used for the next catalytic reaction under similar conditions for up to six cycles.
Due to the bulkiness of the oxindole substrate and reduced microporosity of the CoMOF-2, the authors proposed that the catalytic reaction probably occurs on the surface of the MOF. The densely populated Lewis-acidic metal clusters with a weakly chelated carboxylate group and the Lewis basic site on ligand L (−NH of the amide group) can synergistically activate the epoxide ring and facilitate interactions toward chemical fixation of CO2.
Step 3. Characterize Bioconjugates
With 82% conversion yield and 99% product selectivity, the CoMOF-2/KI combination showed the best catalytic performance at ambient conditions. This may be due to the bulkiness and causes a slower diffusion rate of the other co-catalysts toward the active sites.
Applications Of Epoxide Bioconjugation
Examples of applications of epoxide bioconjugates include their use in the fabrication of graphene oxide, antifungal surface coatings, and protein microarrays.
Application 1. Graphene Oxide Functionalization
Significant interest in carbon nanomaterials during the last decade has led to the great popularity of graphene oxide (GO) functionalization in the scientific community, including the application of GO in the bioconjugation of quantum dots. However, current GO functionalization studies that focus on carbodiimide condensation via carboxyl groups often complicates the modification study for the wide practical application of GO. On the other hand, despite sufficient activity of epoxy groups present during carbodiimide condensation, the potential of epoxy-driven GO functionalization remains sufficiently unrealized (Smith et al, 2019; Song et al., 2014).
In this study, a team of authors proposed the assembly of placing epoxides as the primary functional groups on either side of graphene. Essentially, the epoxide groups will weaken the adjacent C–C bonds and induce structure transformation from honeycomb to diamond-like structure as a result of the hybridization transition from sp2 to sp3. The design had a positive effect on the plasticity while a negative effect on the structure stability—offering different isomeric possibilities for epoxidation of graphene offers exciting frameworks for a multitude of electronic effects to guide the wider application of GO-based devices.
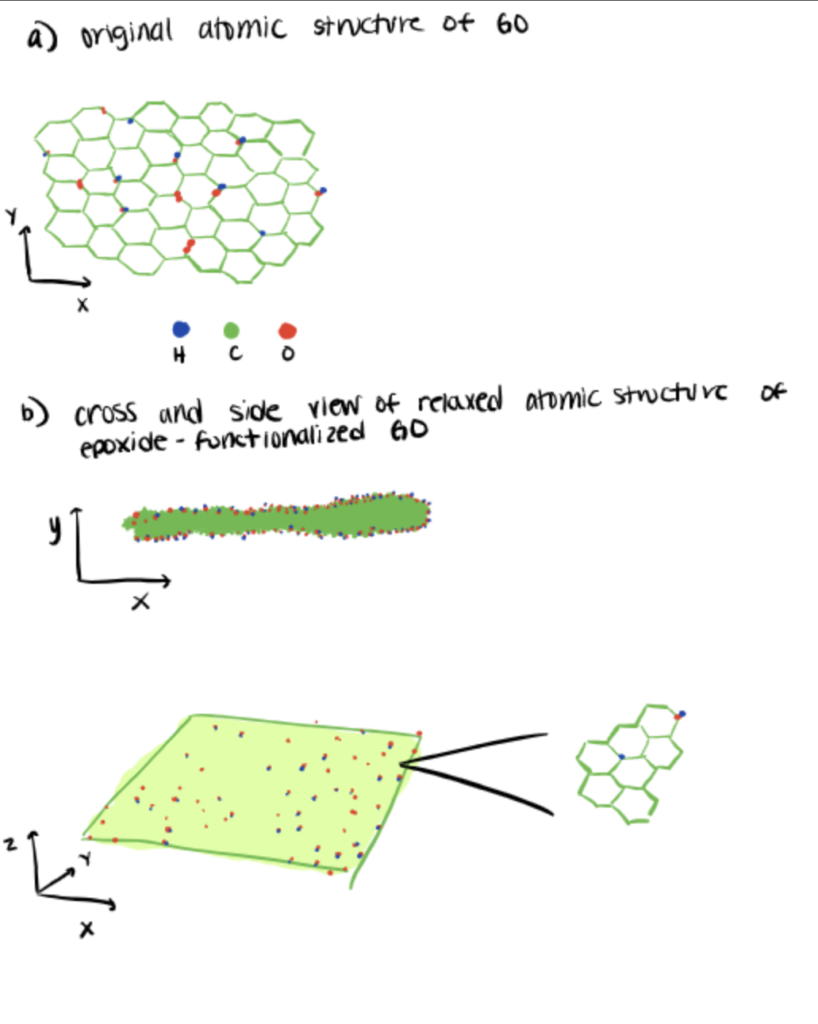
For more information on carbodiimide as a carboxyl group linker, read our article, bioconjugation linkers.
Application 2. Antifungal Surface Coating
Fungal surface colonization and biofilm development on medical devices has long been associated with the manifestation of chronic/systemic infections. In addition to difficulties in the treatment of fungal infections, efforts to release and/or covalently attach antifungal compounds on the device surfaces have been met with various disadvantages (Kojic & Darouiche, 2004; Giles et al., 2018; Michl et al., 2017).
In this instance, one study proposed an attractive strategy to conjugate the antifungal compound onto the surface of the material via an epoxide plasma polymer interlayer. The surface bioconjugation using epoxides is generated via a novel plasma functionalization process that induces a spontaneous reaction between surface epoxide groups and amine groups on caspofungin via an SN2 reaction scheme. The antifungal surface showed excellent solvent stability, withstands detergent washing, and retains activity even after protein fouling. With no by-products to be considered, the process may prove beneficial for scale-up in the industrial manufacture of antifungal surface coatings for medical devices.
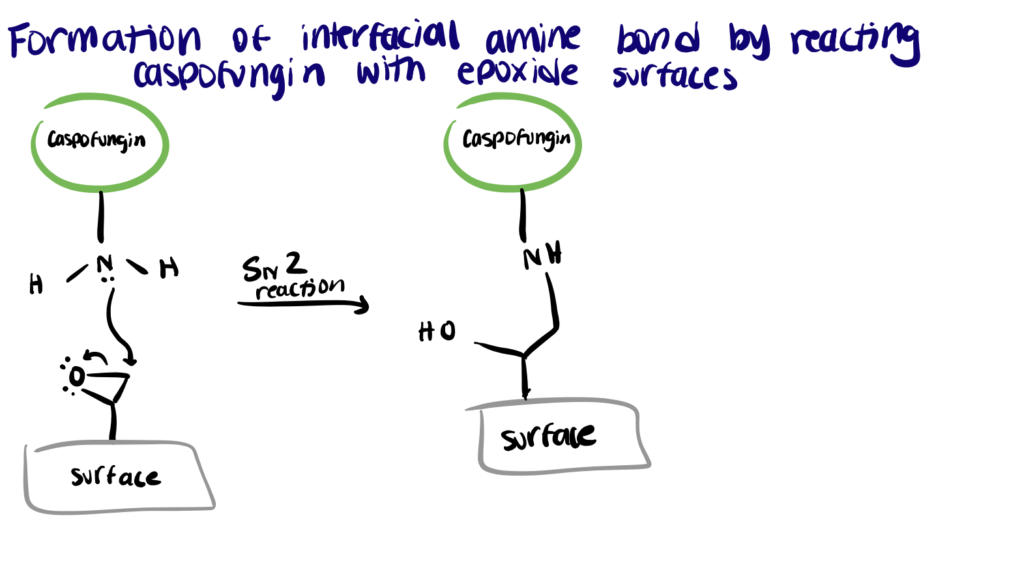
For other methods of antimicrobial surface engineering, read our article on polymer brush bioconjugation.
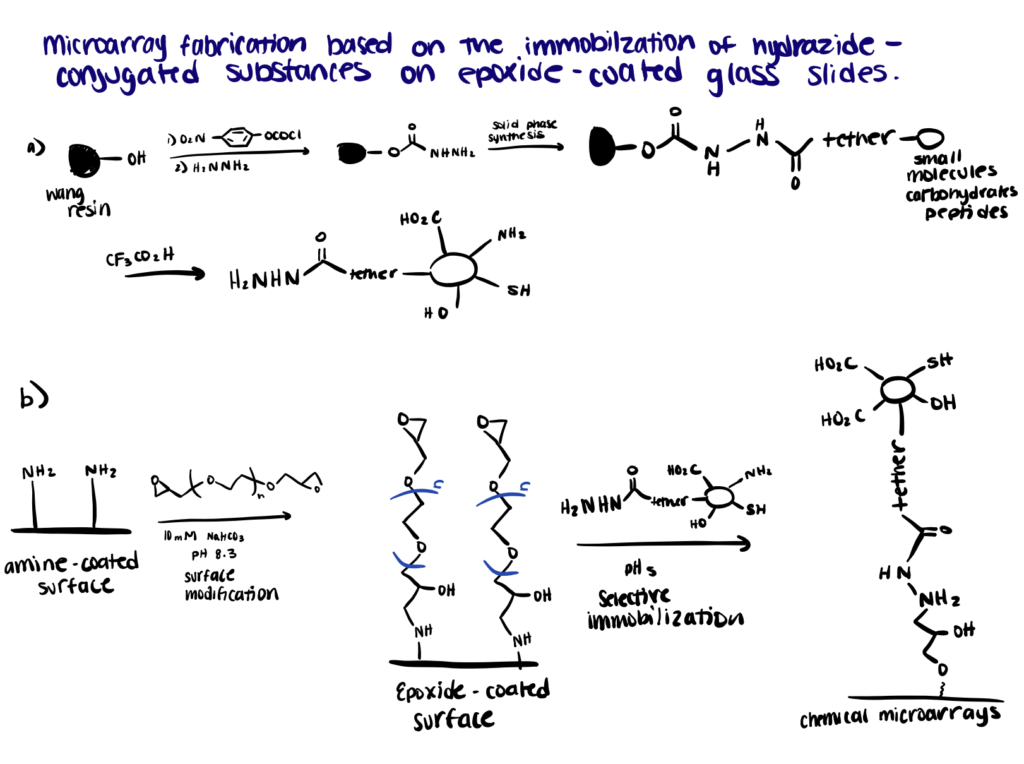
Application 3. Protein Microarray
Recently, protein microarray has received considerable attention as a high-throughput screening tool for proteomics. The major requirement of the applicable techniques for the preparation of microarrays is that the immobilization of the functionalized compounds to the modified surfaces must be highly selective (Smith et al., 2020). One emerging strategy involves the highly selective reaction between hydrazides and epoxides that can take place even in the presence of other potent nucleophiles such as amines and thiols. According to this study, the strategy has proven successful in the construction of microarrays with immobilized hydrazide-conjugated biomolecules on epoxide-coated glass surfaces.