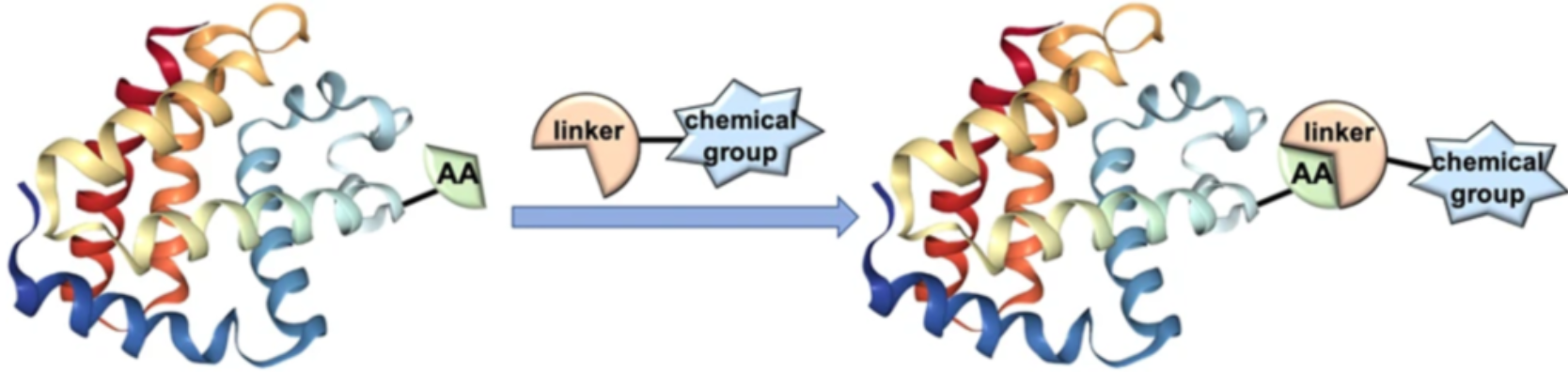
The increased utility of bioconjugate chemistry has advanced the field of chemical biology and therapeutic developments monumentally. Fueled by the recent trends and advances in the field of bioconjugation, scientists have successfully developed a diverse array of chemical bioconjugation methods for complex applications of bioconjugation across multiple disciplines. In this article we’ll discuss electrochemical bioconjugation protocols and applications.
Electrochemical bioconjugation typically involves the cooperation of two radicals with distinct electrochemical roles. This technique can be utilized to attach drugs with antibodies, proteins with polymers, or DNA with metal surfaces.
The vast diversity and intrinsic fragility of biomolecular structures remains a challenge for scientists who are trying to choose optimal linkers for their applications. Potential challenges of various bioconjugation methods, such as regioselectivity, toxicity, structural disruptions, etc. are still often observed with the classical approaches.
In recent years, efforts have moved towards bioorthogonal surface functionalization achieved through single-electron transfer (SET)-based strategies, such as the site-selective electrochemical bioconjugation to tyrosines. Compared to the other traditional and thermal bioconjugation methods, electrochemically-driven bioconjugation is still underexplored.
However, promising research (as we will discuss in this blog) have shown the capabilities of this bioconjugation method to address the issues of selectivity, toxicity, and biocompatibility commonly encountered during common bioconjugation reactions.
What is Electrochemical Bioconjugation?
Electrochemical bioconjugation involves the use of redox chemistry to attach biomolecules together. Here, the oxidation occurs on the surface of the anode, simultaneously with the reduction process occurring on the surface of the cathode.
The reaction involves the cooperation of two radicals exhibiting similar redox potential but contrasting steric demands to suppress both anodic overoxidation of the products and cross-reactivity. Electrochemical bioconjugation significantly improved the selectivity, conversion, and isolated yields—especially for generating cationic reactive intermediates which can be especially useful for arginine bioconjugation, for example, since it is charged at physiological pH.
In contrast to other traditional methods, electrochemical bioconjugation reaction occurs in mild conditions and does not require the use of hazardous chemical reagents. Accompanied with good electrochemical momentum in the field of the oxidative coupling reaction, this anodic oxidation reaction provides attractive rapid (15-75 min), site-selective, biocompatible, mild, and environmentally friendly conditions for constructing novel types of bioconjugates.
More details on electrochemical bioconjugation can be found here.
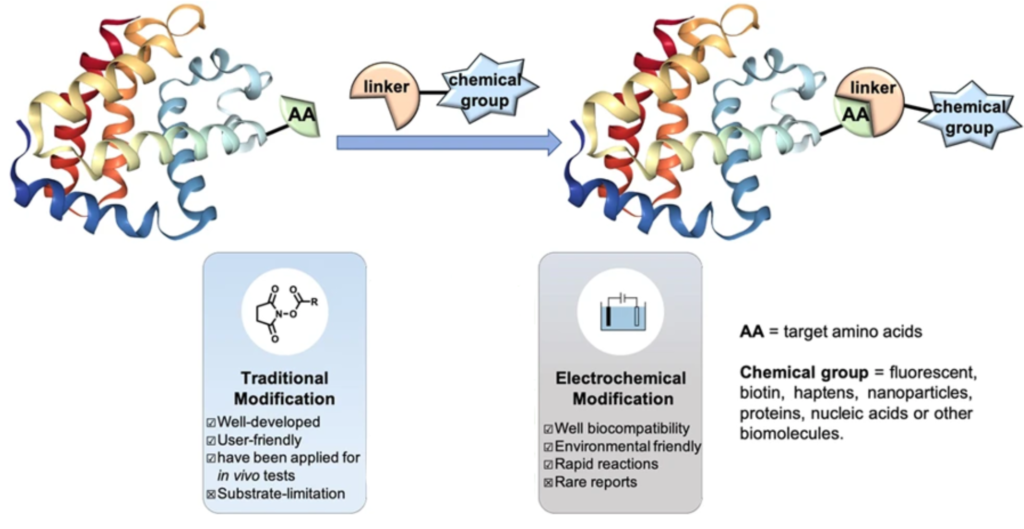
Electrochemical Bioconjugation Protocols
Although there is no extensive research available, recent years have seen the development of several modern strategies for controlling electrochemical bioconjugation as alternatives to the more commonly used strategies.
Electrochemical bioconjugation protocols for tryptophan involve preparing the reaction mixtures, then conducting electrolysis, and finally purifying the conjugates. For direct electrochemical bioconjugation on a metal surface, functionalized monolayers are first assembled then conjugated with DNA.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Electrochemical Tryptophan-Specific Bioconjugation
Reports of electrochemically-induced tryptophan bioconjugation are rare. However, Toyama et al., (2019) had successfully developed one interesting strategy to promote tryptophan-specific electrochemical bioconjugation.
In an effort to optimize their previous research using chemical activation, the authors successfully conducted a transition metal-free tryptophan-specific bioconjugation reaction in neutral aqueous media and increased target product yield (from 33% to ≥75%)—simply by applying electric current to the reaction solution.
Step 1. Prepare Electrochemical Bioconjugation Reagents
To prepare the protein (lysozyme and bovine serum albumin), keto-ABNO, and 4-oxo-TEMPO radical mixtures, the authors dissolved the compounds in a 0.1 M LiClO4 aqueous solution.
Step 2. Electrolysis Reaction
The mixture was electrolyzed using graphite and platinum as the working and counter electrode, respectively. A full conversion of lysozyme was observed by the authors at a constant voltage of 1.0 F/mol electric charge at 0.9 V. In the case of bovine serum albumin, approximately 70% conversion was observed at a constant voltage of 1.0 F/mol electric charge at 1.0 V.
Step 3. Cyclic Voltammetry
Cyclic voltammetry measurements were conducted by the authors to gain preliminary insight into the reaction mechanism. Both radicals did not interact with each other in the absence of tryptophan residues. However, in the presence of tryptophan, a distinguishable irreversible bioconjugation reaction proceeded between keto-ABNO and the amino acid residue. Notably, no interaction was observed between the 4-oxo-TEMPO radical and the tryptophan complex—possibly due to steric hindrance.
Step 4. Purification of Bioconjugates
The authors didn’t specify how they purified the bioconjugates, but various methods for isolation can be found here.
Electrochemical Oxidation Induced Tyrosine-Specific Bioconjugation
The naturally low abundance of tyrosine residues on native protein surfaces—and their tuneable reactivity by selective deprotonation—highlights the advantages regarding tyrosine as a labeling target in electrochemical bioconjugation systems. We’ve discussed several tyrosine bioconjugation techniques in another article.
One successful electrocatalytic modification of tyrosines was reported by Song et al., (2019). The authors proposed a promising strategy with high chemoselectivity and site-selectivity to label tyrosine residues with phenothiazine derivatives under mild conditions—producing up to 85% of the yield product.
Step 1. Cyclic Voltammetry
Similar to the previously discussed protocol, the authors first performed cyclic voltammetry experiments of 0.005 M of the phenothiazine, tyrosine, tryptophan, phenylalanine, and histidine amino acids at a glassy carbon electrode (0.05 M nBu4NBF4 in CH3CN/H2O) to gain insight into their relative redox activity.
The results showed that the oxidation potential of phenothiazine was much lower than that of the other corresponding amino acids—indicating that the reaction proceeded through the single-electron oxidation of phenothiazine by the anode to generate the nitrogen radical species.
Step 2. Electrolysis Reaction
The electrolysis reaction was conducted in a three-necked undivided cell with a graphite rod anode and a nickel plate cathode at 10 mA constant current for 75 minutes, with Na2SO4 as the electrolyte in CH3CN/H2O (pH 7.3, room temperature).
The generated free radical was then added to the ortho-position of phenol to obtain the labeled tyrosine. Oxidation of the other amino acids in the protein would not be favored due to their less electron-rich positions.
Step 3. Extraction Of The Labeled Proteins
When the reaction was finished, the reaction mixture was extracted with ethyl acetate. The combined layers were dried over Na2SO4 and concentrated. The pure product was obtained by flash column chromatography on silica gel (dichloromethane:methanol ¼ = 100:1). One other potentially interesting application of this method is bioconjugation of quantum dots to tyrosine residues for ultrasensitive imaging.
Electrochemical Bioconjugation of DNA to a Gold Surface
For many years, the unique capabilities of DNA-gold bioconjugates have fostered their use in various fields of research, ranging from the prevention against food fraud, labelling of biomolecules, selective biosensors, and many more.
There are many methods for bioconjugation of DNA to produce DNA-biomolecule conjugates, as discussed previously in our blog. However, many of the methods are either not yet tested on larger proteins/oligonucleotides, require redox-active catalysts, or they are hindered by a lack of control over the secondary interactions between the nucleobases and metal surfaces. This complicates the formation of a well-defined homogeneous monolayer with adequate density.
Furst et al., (2017) highlighted the use of electrochemical bioconjugation as a simple, sensitive, fast, accurate, and inexpensive tool for the generation of DNA-gold bioconjugates. Using catechol functionalized gold surfaces, the author demonstrated that the electrochemically activated conjugation method generates ssDNA monolayers in a matter of minutes without the use of additional reagents. If you’d like other thiol-mediated bioconjugation methods, read our related article.
Step 1. Functionalization Of Monolayers
The authors first functionalized the monolayers on the catechol-coated gold electrodes that were oxidized to o-quinones via oxidative bioconjugation to allow interaction with anilines introduced on the DNA strands.
Preliminary studies indicated that the nitro groups of thiols 1 and 2 as precursors to o-iminoquinones and o-quinones generated in situ could be reduced smoothly to anilines. Sufficient dilution of the o-nitrophenols on the gold surface should be properly conducted to prevent self-coupling of the oiminoquinones with o-aminophenols that had not yet been reduced.
Step 2. Monolayer Assembly
Following H2SO4 electrode cleaning, monolayers were assembled on the surfaces of gold rod electrodes by exposure to ethanolic solutions of the thiol mixtures (a combination of thiol 1 and mercaptoethanol) at RT for 12-18 h. The electrodes were scanned in 10 mL of DPBS to evaluate the formation of the monolayers.
Step 3. Synthesis Of Dna-Coupling Partners
DNA was modified with an NHS ester, followed by azide reduction with TCEP, as previously reported by Palla et al. (2016) to ensure coupling to the cell surfaces
Step 4. DNA Modification On Gold Surfaces.
A drop of aniline-modified DNA in PBS (pH 7.2) was placed on the center of the electrode. Constant potential amperometry at a potential of 0.35 V for 240 s was used to activate the catechol-modified surfaces for aniline-DNA attachment. Following the application of a potential, electrode surfaces were rinsed with PBS and Nanopure water.
Step 5. Quantification Of DNA On Electrode Surfaces
DNA-modified electrodes were subjected to electrochemical measurement with 20 μM ruthenium hexamine, which electrostatically interacts with the phosphate backbone of each strand, diluted in Tris buffer (0.1 M pH 7.6). Cyclic voltammetry scans were obtained at a scan rate of 100 mV/s.
An alternative method is to first complex DNA with protamine and then to conjugate functional groups of protamine onto the surface. Read about protamine bioconjugation in our related article.
Applications That Utilize Electrochemical Attachment Techniques
Electrochemical bioconjugation is also often termed as a ‘green’ or ‘clean’ bioconjugation strategy, as it eliminates the requirements of hazardous metals, oxidants, and/or additives. By preventing harsh reaction conditions and/or reactants, electrochemical bioconjugation could significantly enrich the methodologies of bioconjugation chemistry in the field of chemical biology, medical chemistry, and clinical pharmacology.
Some applications of electrochemical bioconjugation include tumor imaging, fabrication of nanostructures, and hydrogen construction.
Application 1. Tumor Imaging
It is well-known that targeting amino acid residues present on lower abundance on protein surfaces would lead to a higher degree of bioconjugation specificity. In this instance, electrochemical bioconjugation techniques play a central facilitating role to facilitate the production of supernatural protein functions that are not easily accessible by complementary methods which utilize genetic manipulations.
Sato et al., (2020) recently reported on an electrochemically activated site-specific modification on the HC–Tyr57 site of trastuzumab for in vivo imaging and construction of antibody-drug conjugates without gene manipulation. It should also be noted that the addition of HRP as a catalyst increased the efficiency of the reaction.
Related articles:
- You might also consider bioconjugation via maleimide for installing labels onto proteins in a highly specific manner
- As an alternative, FITC labeling is a commonly method, which is selective for amine-containing proteins
Application 2. Fabrication Of Nanostructures
Electrochemical bioconjugation has been successfully adopted for the preparation of various nanostructures for applications in various areas. In 2006, Ramanaviciene et al. utilized the electrochemical preparation of polypyrrole-based artificial receptors for caffeine detection. Following this, Ghorbal et al. (2013) also demonstrated nano-electrografting of non-conducting organic polymers onto conducting surfaces—representing another step towards improved nano-processes for nano-electrografting.
Interestingly, electrochemical bioconjugation has also received great interest as a pretreatment or advanced treatment process for wastewater management. As reviewed by Dionisio et al., (2021) and Hu et al., (2020), electrochemical bioconjugation may serve as a powerful method for the removal of toxic pollutants from water in the future.
We have other articles on the fabrication and application of nanostructures, bioconjugation of nanoparticles and magnetic nanoparticle bioconjugation.
Application 3. Construction Of Hydrogels
Existing for more than half a century, hydrogels are now gaining more popularity as mediums for various applications—ranging from agriculture, food industry, pharmaceutical field, and biotechnology. Hydrogels are commonly prepared via chemical and/or physical cross-linking, as summarized by Ahmed (2015).
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Recently, the electrochemical bioconjugation approach has received much attention in the formation of hydrogel constructs. Unlike gels formed in bulk which take the shape of the container, they are poured into when liquid, electrochemically fabricated hydrogels can be formed directly onto an electrode surface or any conductive surface. This provides a high level of programmable spatiotemporal assembly to encapsulate enzymes, nanomaterials, drugs or cells (Cross, 2020; Dhanjai, 2019).
An increasing number of publications have reported the successful formation of calcium-alginate hydrogels for various applications (Ozawa et al., 2013, 2013, 2016; Wang et al., 2011; ). This method involves inserting electrodes into a sodium alginate solution containing CaCO3 particles. The H+ generated near the electrode by the electrolysis of water will react with the CaCO3 particles to release Ca2+ into the sodium alginate solution—resulting in the deposition of calcium-alginate hydrogels on the electrode surface.
