Interest in tyrosine bioconjugation is increasing rapidly because of the vast range of applications that tyrosine-specific conjugation can enable. Tyrosine bioconjugation can be utilized for applications such as studying biological systems using super-resolution microscopy, flow cytometry, and functionalizing proteins with fluorescent biosensors to detect the activity and molecular signals in living cells. In this article we’ll discuss different methods of tyrosine bioconjugation.
Different tyrosine bioconjugation methods include Mannich-type reactions, reactions with diazonium reagents, diazodicarboxyamides, transition metal-mediated approaches, sulfur-fluoride or triazole exchange, and the chemical O-glycosylation of tyrosine.
What Is Tyrosine?
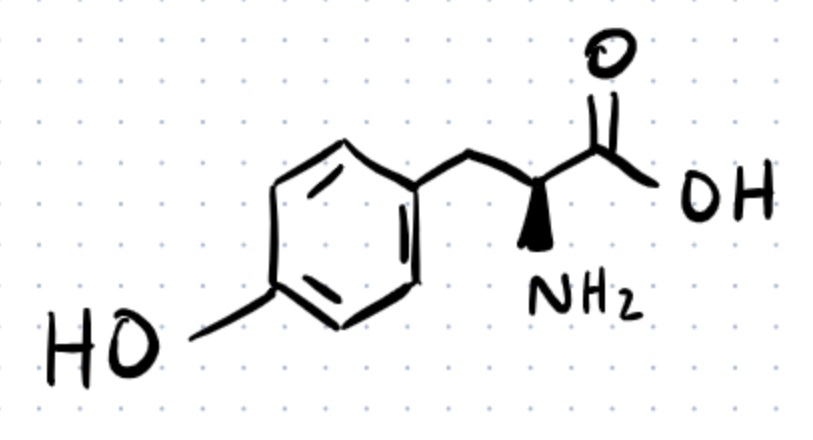
Tyrosine is a non-essential amino acid produced by the body by metabolizing phenylalanine. It is required for the formation of several critical neurotransmitters, such as dopamine, epinephrine, and norepinephrine.
Tyrosine also aids in the production of melanin, the pigment that gives hair and skin their color. It’s present in almost every protein structure in the body and it supports the adrenal, thyroid, and pituitary glands, which are responsible for producing and regulating hormones. Learn more about it here.
Tyrosine Bioconjugation Methods
The advancement of chemical biology and the requirement for more homogenous protein conjugates have fueled vigorous research on new bioconjugation techniques over the last two decades. To functionalize the phenol side chain of tyrosines, a variety of bioorthogonal labelling techniques have been reported. Tyrosine residues are rather common and largely hidden at the protein surface, providing intriguing possibilities for site-specific labelling of these reactive amino acids.
Methods for tyrosine bioconjugation include mannich-type reactions, using diazonium reagents, diazodiaceboxyamides, transition metal-mediated approaches, triazole exchange, and chemical o-glycosylation.
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
Tyrosine targeting has been successful in the development of a variety of critical biomolecules, including PEG- conjugates, radioactive or fluorescent protein probes, glycoengineered vaccines, protein aggregates, and antibody-drug conjugates. Innovative approaches for site-specific labelling with ligand-directed anchoring and particular affinity capture of proteins have also been reported. Here’s a great review by Dorta et al. in the Chemistry Journal.
Alternative approaches compared to bioconjugation to tyrosines include bioconjugation of maleimides to cysteine amino acids and lysine conjugation protocols. There are also other thiol-mediated bioconjugation methods available besides maleimide chemistry.
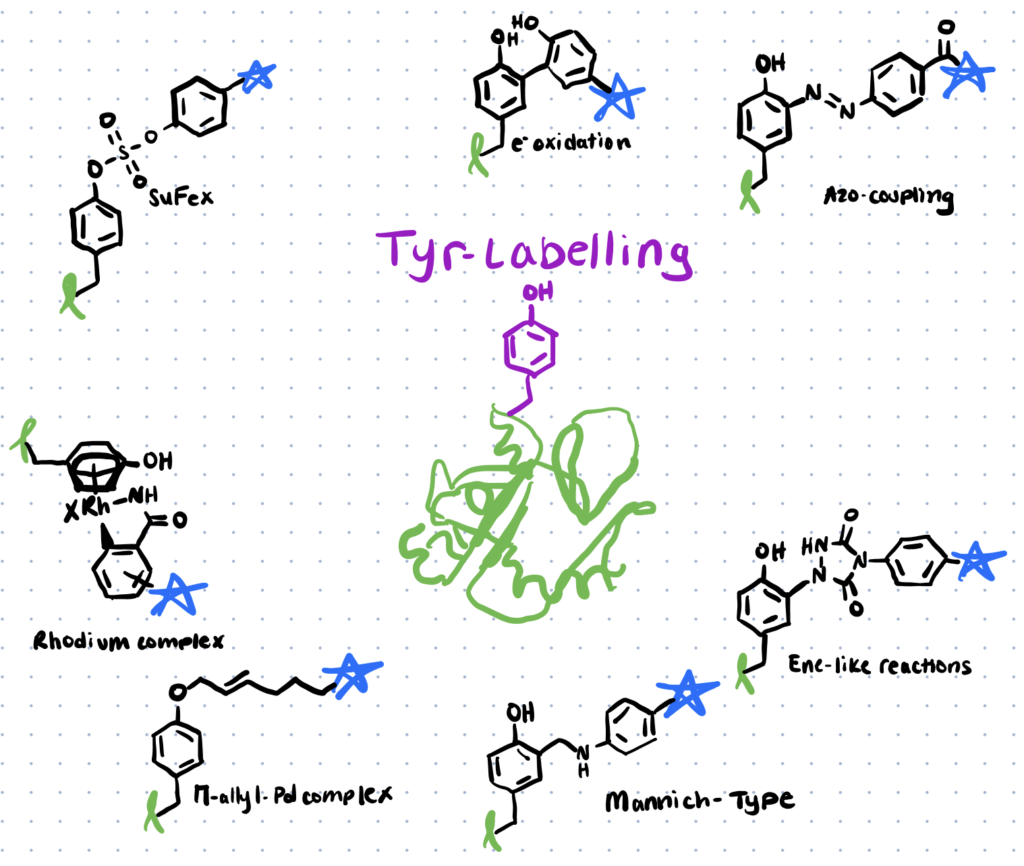
Here is an in depth look at the six different methods of tyrosine bioconjugation:
Method 1. Mannich-type Reactions
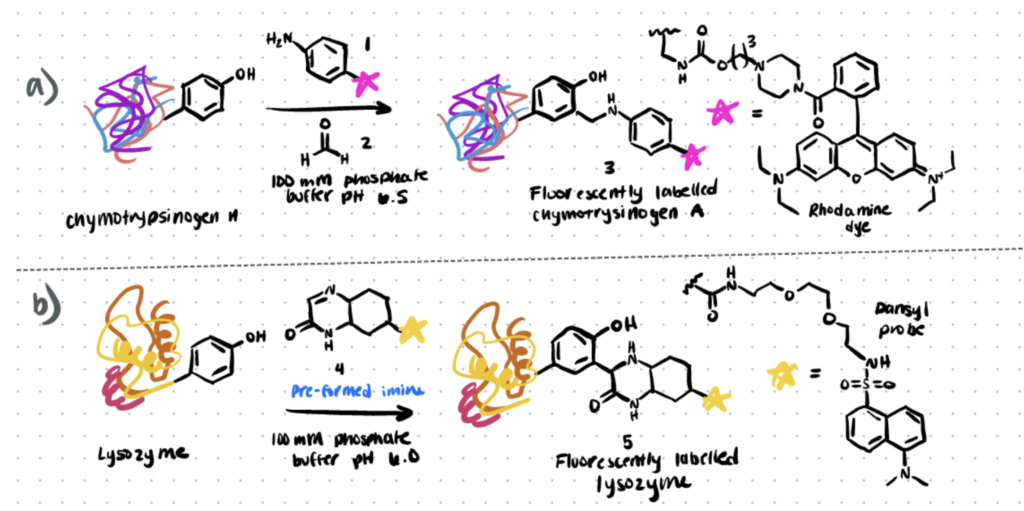
Mannich-type reactions were among the earliest procedures for tyrosine modification to be developed (Scheme 1). The three-component Mannich-type reaction, in this case, comprises the synthesis of an imine from a modified aniline (compound 1 below) with an aldehyde (compound 2 below), which then interact with the tyrosine to create a carbon–carbon bond, forming the tyrosine bioconjugate (compound 3 below). (Scheme 1a). Fluorophores and synthetic peptides are attached to chymotrypsinogen using this process.
Method 2. Diazonium Reagents
The process of coupling diazonium derivatives to (poly)peptides has been used for over a century. These substrates are useful for protein labelling because of their strong reactivity.
The field of diazonium coupling has detailed the modification of tyrosine residues on bacteriophage MS2 viral capsids and tobacco mosaic virus capsids in various papers (TMV). Since then, Wang et al. have published a work on the functionalization of TMV using diazonium coupling at tyrosine, which has also been used to change the tyrosine residues of the M13 bacteriophage (source).
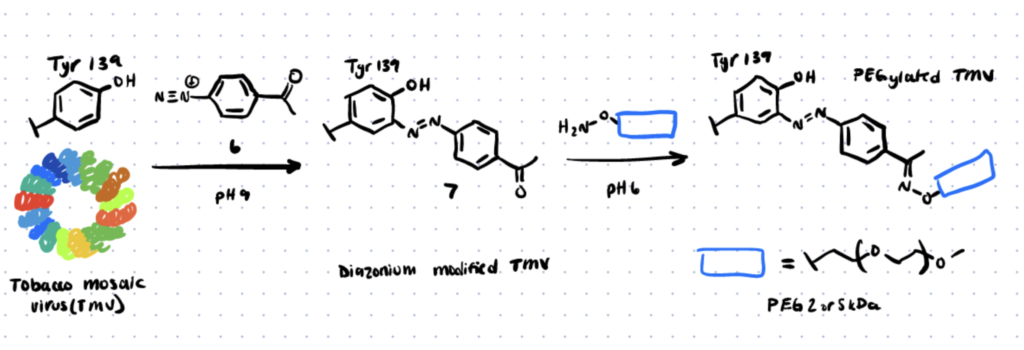
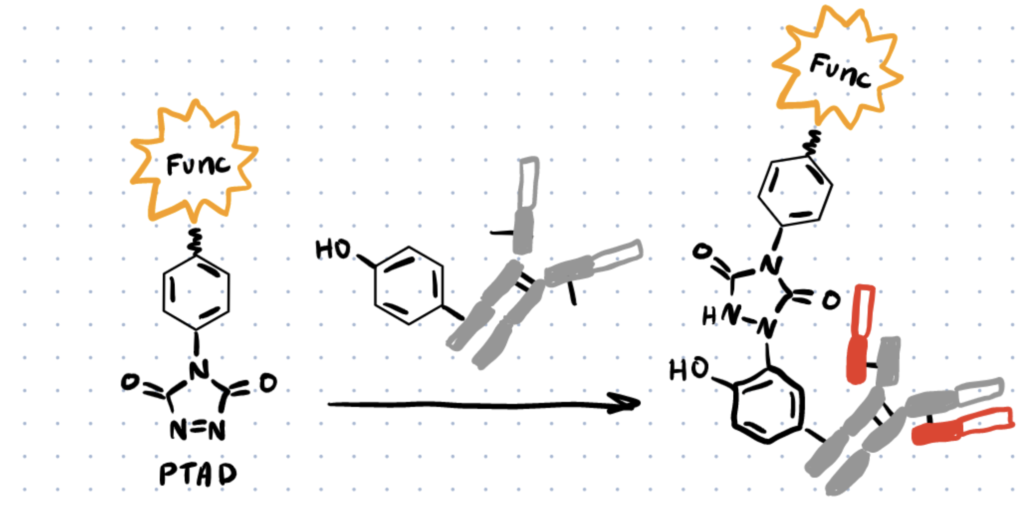
Method 3. Diazodicarboxyamides
Ban et al. discovered one of the most extensively used ways for tyrosine modification, which uses cyclic diazodicarboxyamides, such as 4-phenyl-3H-1,2,4-triazoline-3,5(4H)-diones (PTADs), which can be made from their precursors (molecule 8 below) via chemical oxidation using reagents like molecule 9 below (scheme 3). These compounds react faster than previously established reagents, with reaction durations ranging from 15 to 30 minutes, and are selective for tyrosine until breakdown occurs.
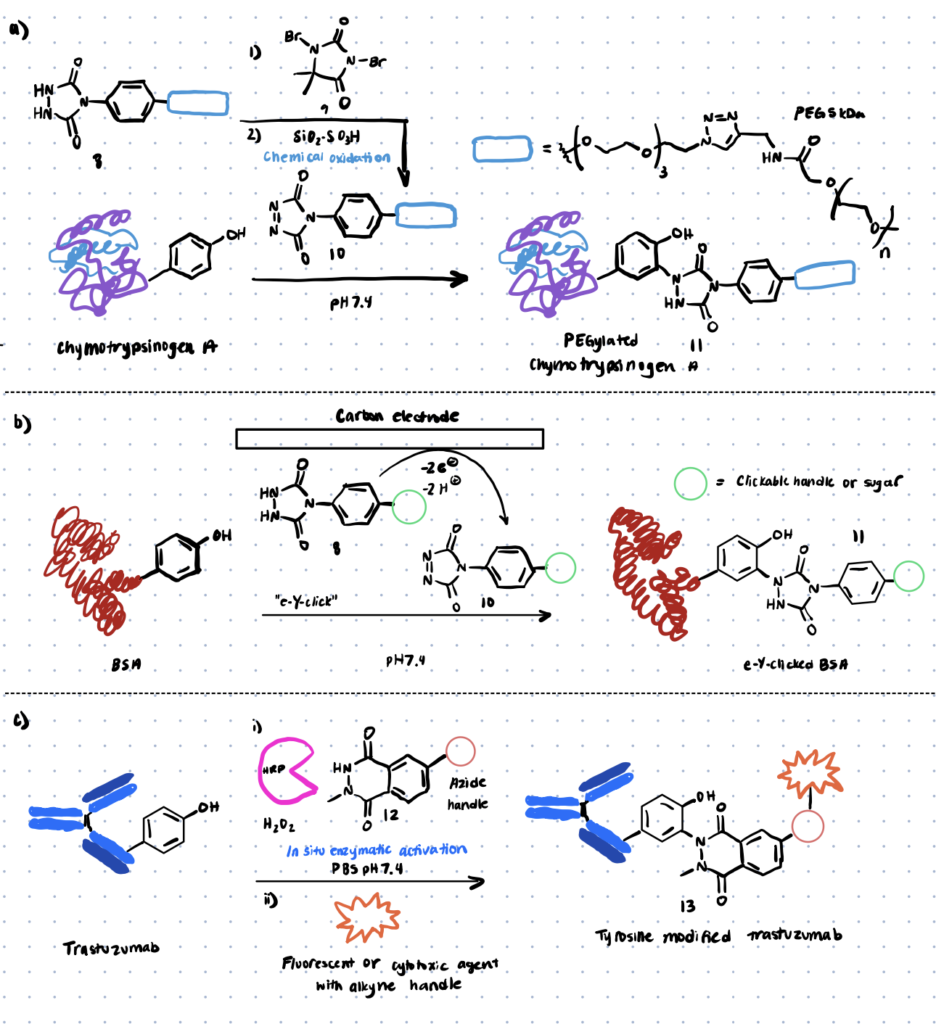
Method 4. Transition Metal-mediated Approaches
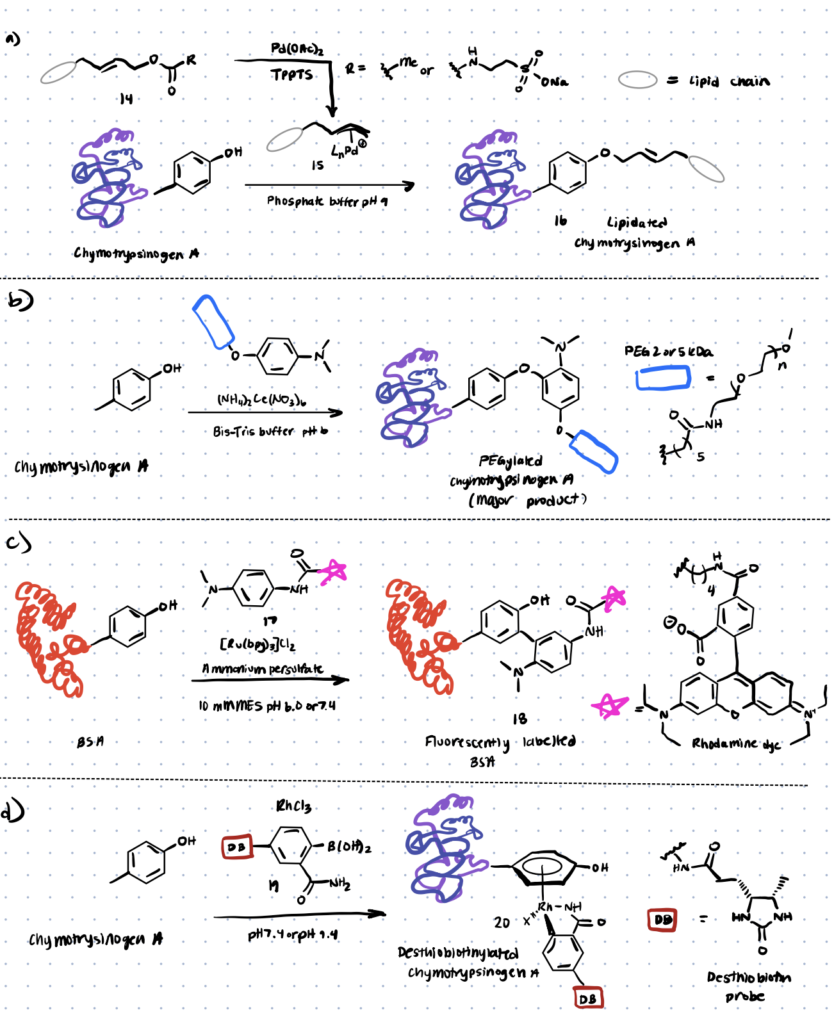
For tyrosine modification, a variety of transition metal-mediated methods are known. Previously, Tilley et al. investigated how tyrosine reacts with an electrophilic -allyl palladium complex (compound 15, Scheme 4), resulting in tyrosine O-alkylation. In the presence of palladium (II) acetate, the complex is formed from allylic acetate (compound 14 below). This technique resulted in a 50–65 percent conversion (monoadduct) of chymotrypsinogen in 45 minutes at pH 8.5–9.
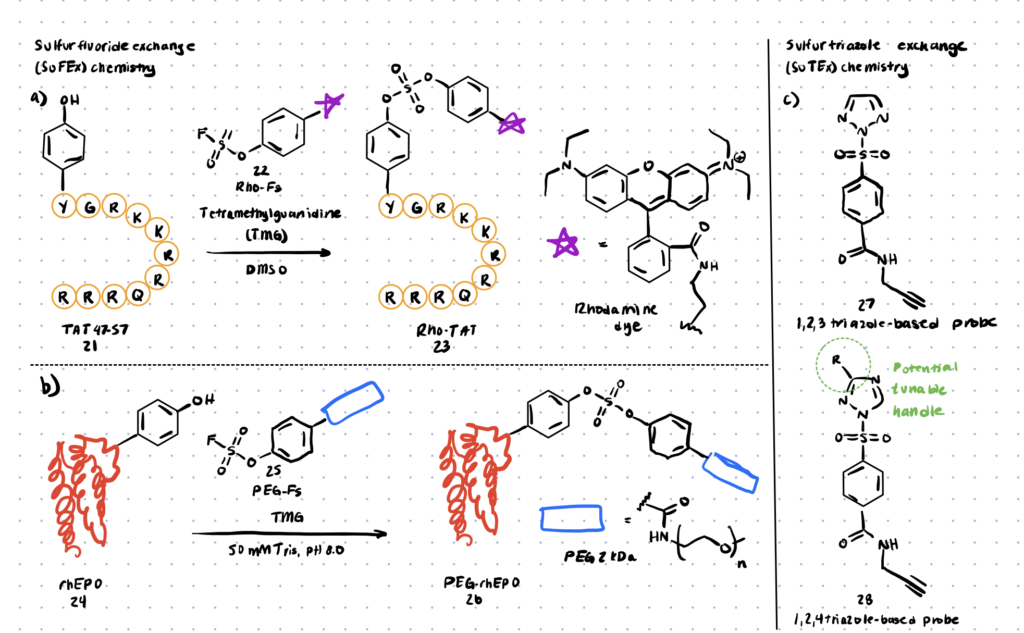
Method 5. Sulfur-fluoride Or Triazole Exchange
In articles like this one, sulfur fluoride exchange (SuFEx) chemistry has recently been characterised for its potential in click chemistry and can be used to modify tyrosine residues in cell proteome analysis. According to the article, this particular method of click chemistry is more advantageous as it is highly reactive to nucleophiles under the right circumstances, is unaffected by water and oxygen, and is UV and thermally inactive.
Method 6. Chemical O-glycosylation Of Tyrosine
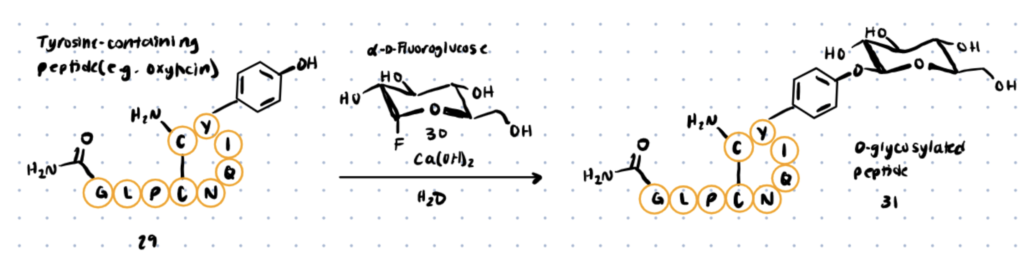
Using fluoro glycosyl donors, a new approach for synthetic O-glycosylation of tyrosine-containing peptides has recently been disclosed (link to paper). Unlike previously described methods, this procedure allows for phenolic O-glycosylation in aqueous conditions without the use of a protective group.
To begin, Boc-LTyr-OH was O-glycosylated with -D-fluoro glucose (compound 30 below) in the presence of various additives in aqueous conditions. Ca(OH)2 helped produce the desired O-glycosylated product (compound 31 below) with a 94 percent conversion within 10 minutes.
For another application of bioconjugation that utilizes glycosylation, read our article, nanobody bioconjugation.
Site-Selective Tyrosine Bioconjugation
Tyrosine is present in numerous polypeptides and proteins, including tyrosine protein kinases, kisspeptin, and myoglobin. It is an interesting target for labelling biomolecules because of its low natural abundance in native proteins. The benefit of modifying naturally low abundance aromatic amino acid residues leads to a higher degree of bioconjugation without altering the overall charge state or redox sensitivity. You can read about several oxidative coupling-based methods for site-selective protein modification at tyrosine and other residues in the article.
Methods that can be used to achieve site-selective tyrosine bioconjugation include photoredox catalysis, electrochemical oxidation and iminoxyl radicals.
Related articles:
- NHS ester bioconjugation techniques can be used to react with amines on lysine amino acids
- FITC labeling, which involves the use of an isothiocyanate group to react with amines on lysines, is a common technique as well
Photoredox Catalysis
While genetic engineering can make recombinant proteins with non-natural amino acids, regioselective chemical modification of wild-type proteins is still a difficulty.
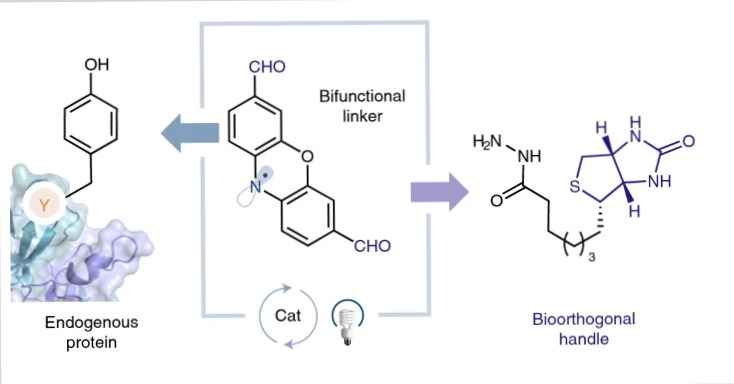
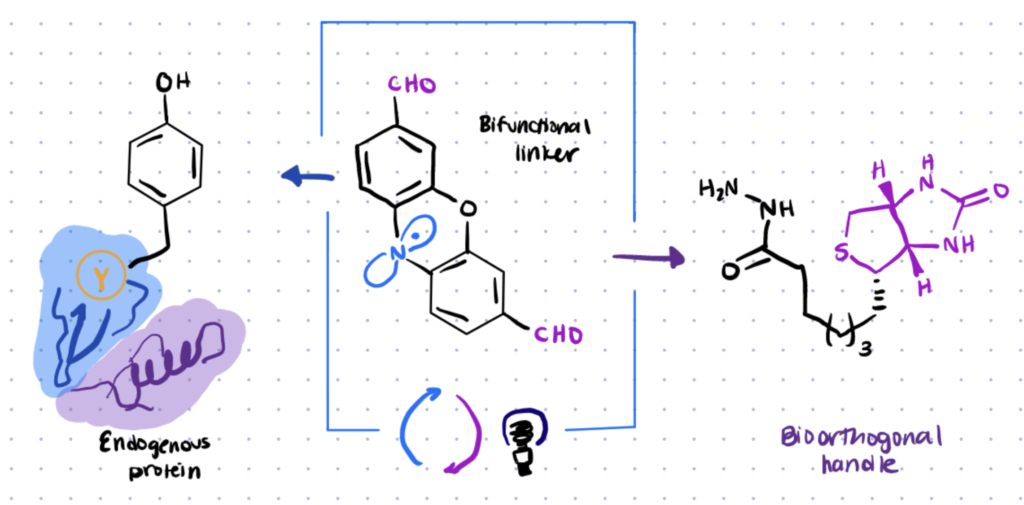
Photoredox catalysis can be used for site-selective tyrosine bioconjugation along with bioorthogonal formyl groups. This can enable you to make structurally specific fluorescent conjugates from natural proteins.
Lumiflavin, a water-soluble photocatalyst, was previously utilized to conjugate a phenoxazine dialdehyde tag with a single tyrosine on a protein, even in the presence of several tyrosines.
Both tyrosine-specific and single-site-selective labelling may be done effectively on a range of native proteins using the lumiflavin method, including those having multiple tyrosines. For more site-selective labeling methods, check our article on histidine bioconjugation.
Once the lumiflavin inserts aldehyde moieties directly onto natural proteins, you can then utilize a variety of well-known bioorthogonal functionalization procedures to couple other biomolecules to the protein such as hydrazines.
Electrochemical Oxidation
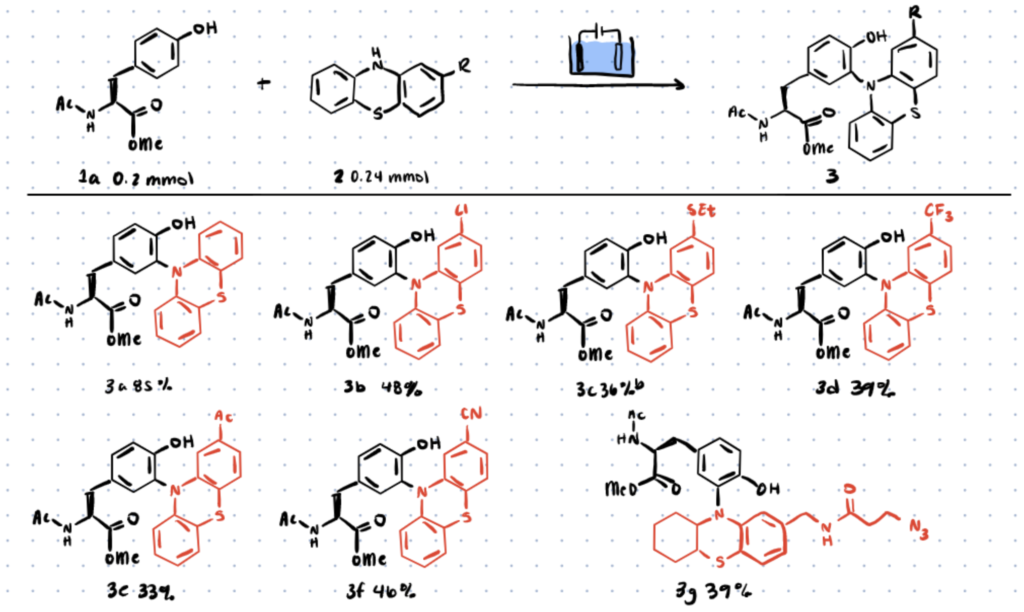
In previous work, protected tyrosine (compound 1a, below) and phenothiazine (compound 2a, below) were chosen as model reaction substrates for optimization in order to establish acceptable conditions for electro-oxidative bioconjugation to tyrosines.
The researchers carried out the reaction in a three-necked undivided cell with a graphite rod anode and a nickel plate cathode at 10 mA constant current for 75 minutes under electrolysis conditions.
Different electron donating or electron withdrawing substituted phenothiazines were discovered to react with different tyrosines under these reaction conditions.
Iminoxyl Radicals
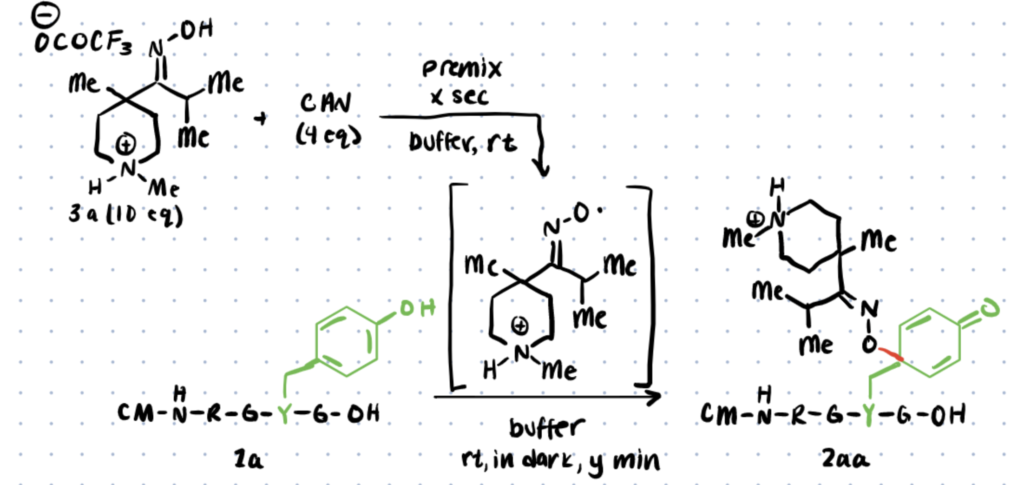
Tyrosine (Tyr) is a low-reactive proteinogenic amino acid with a modest natural abundance (3-4%) and surface exposure in primary protein sequences, as stated in this report.
In the article, the authors describe how iminoxyl radicals could react, site-specifically with tyrosine residues, under biocompatible circumstances. The reaction was conducted at room temperature, in buffered media, and under air!
The conjugate stability could be adjusted based on the structure of the iminoxyl radicals. The aforementioned report gives a detailed explanation of how iminoxyl radicals enable tyrosine-selective protein bioconjugation.
Tyrosine Bioconjugation Applications
Tyrosine bioconjugation applications include biotherapeutic, bioanalytical, and vaccine applications.
Biotherapeutic Applications
Antibody–drug conjugates are an important class of medicines, particularly in cancer treatment (also referred to as ADCs). These structures combine the cytotoxicity of small-molecule medicines with the specificity of monoclonal antibodies, allowing for targeted administration while minimizing side effects and general toxicity.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
Tyrosine bioconjugation is possible to create antibody drug conjugates by using PTADs (4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione), which are a cyclic diazodicarboxamide derivative. (From Creative Biolabs).
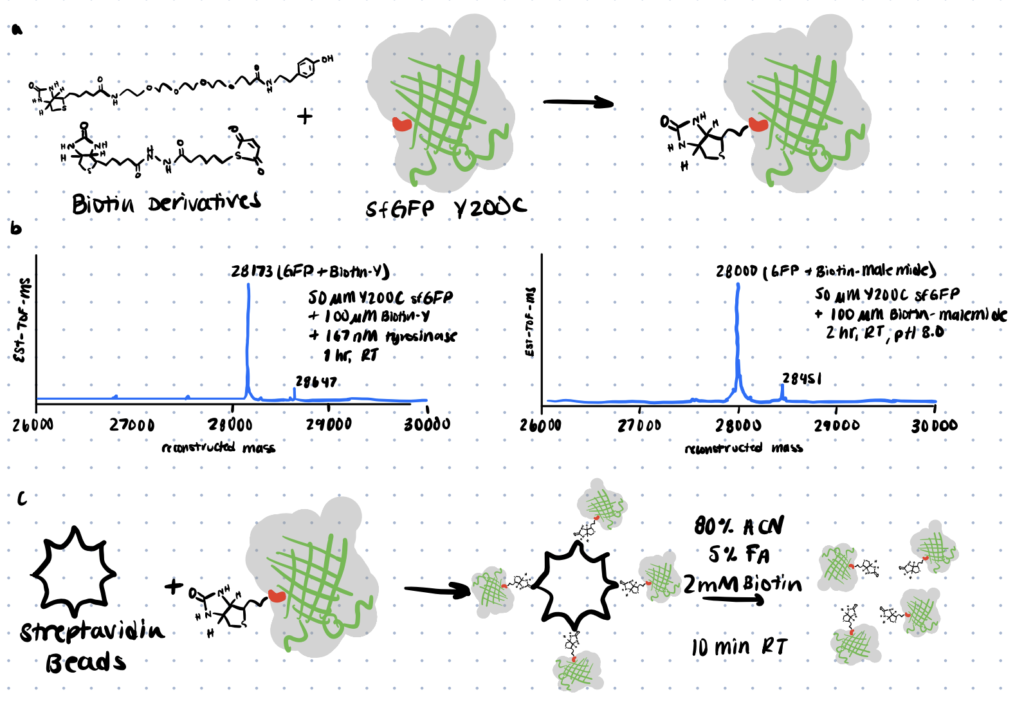
Bioanalytical Applications
In this article, the authors showcase how tyrosine conjugation can be utilized for protein-protein and protein-peptide bonding. In the article, Y200C sfGFP was attached to a biotin moiety using either maleimide or tyrosinase-based conjugation and then stability tests were performed in human blood serum.
The maleimide-coupled product diminished over time and was no longer identifiable in either sample after 7 days. The tyrosinase-coupled product, on the other hand, was retrieved throughout the experiment, demonstrating that it had far greater serum stability.
Bioconjugation to quantum dots is another potential strategy for analyzing samples containing tyrosine
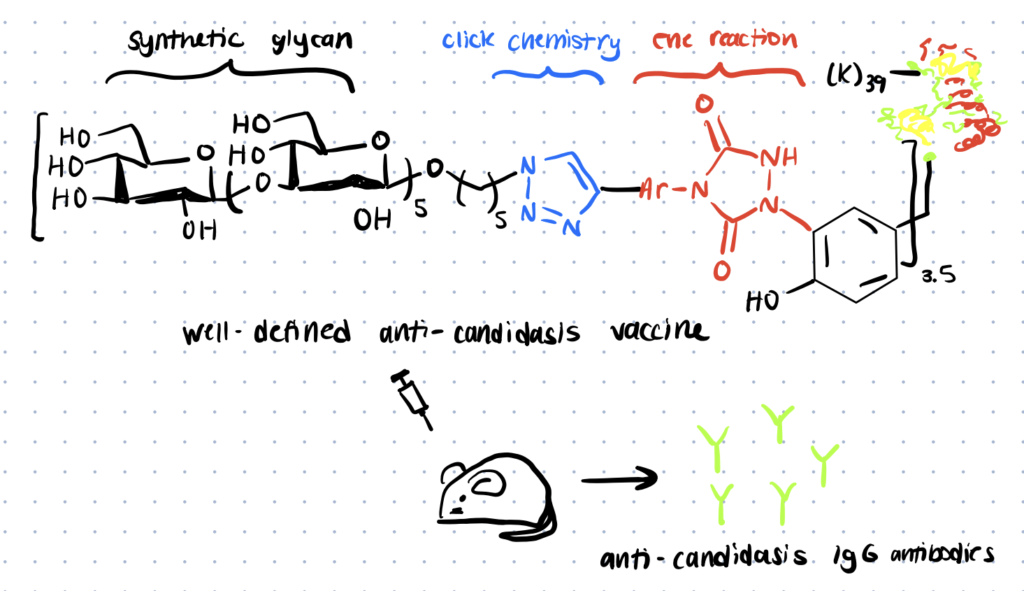
Vaccine Applications
A tyrosine-selective alkynylation and a click chemistry-mediated glycoconjugation sequence were used to create glycoconjugate vaccines in this article. A well-defined glycan, protein carrier, and connection are all present in this conjugate.
Protocol For Site-selective Tyrosine Bioconjugation
Here’s a general method for site-selective tyrosine bioconjugation. You can find the full method in this article. In the work, a surrogate substrate, Thymopentin, containing lysine and tyrosine was used to enable the use of NMR.
Step 1. Incubate Tyrosine Peptide with phenyl-1,2,4-triazoline-3,5-dione (PTAD)
Thymopentin was treated overnight at room temperature with PTAD (1.1 eq.) in Tris buffer (pH 7.4). This limits reactivity towards lysines.
Step 2. Incubate with a Modified PTAD
Once you can prove that PTAD works with Tyrosine on your peptide or protein, use a modified PTAD such as compound 3 below to introduce click functionality onto the tyrosine residues.
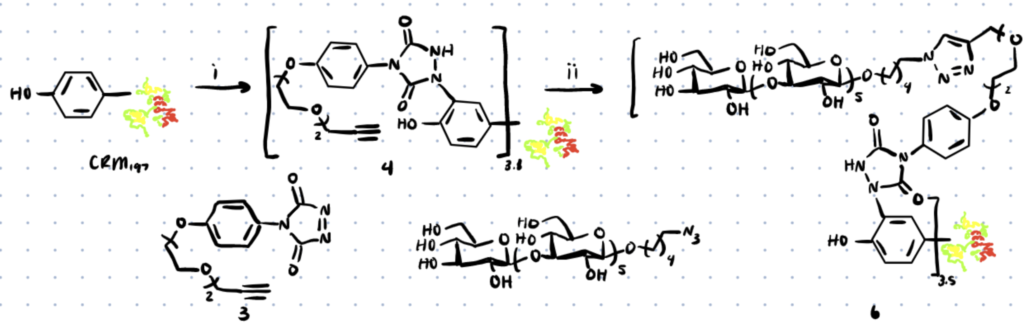
Step 3. React with any Azide Containing Molecule
Now that the alkyne is present on the protein, you can use click-chemistry to react with it. We’ve discussed orthogonal bioconjugation methods in more detail here.
