The tremendous applicability of nanoparticle science is rapidly changing the landscape of various scientific fields and defining new technological platforms. This is perhaps even more evident in the field of nanomedicine whereby nanoparticles (nanoparticles) have been used as a tool for the treatment and diagnosis of many diseases. The way by which nanoparticles enter the cell is a key factor in determining their intracellular fate, corresponding biological response, and therapeutic effect. Over the past years, considerable numbers of studies have been conducted to understand the mechanisms of NP-cell interactions to further advance the field. In this article we will discuss bioconjugation and cellular uptake and how to modulate cellular uptake by conjugating different molecules together.
Different cellular uptake mechanisms include clathrin-mediated endocytosis, caveolin-mediated endocytosis, receptor-mediated endocytosis, and macropinocytosis. Bioconjugation to chitosan increases clathrin-mediated endocytosis, hyaluronic acid bioconjugation increases caveolin-mediated endocytosis, and folate bioconjugation increases folate receptor-mediated endocytosis.
Cellular Uptake Mechanisms
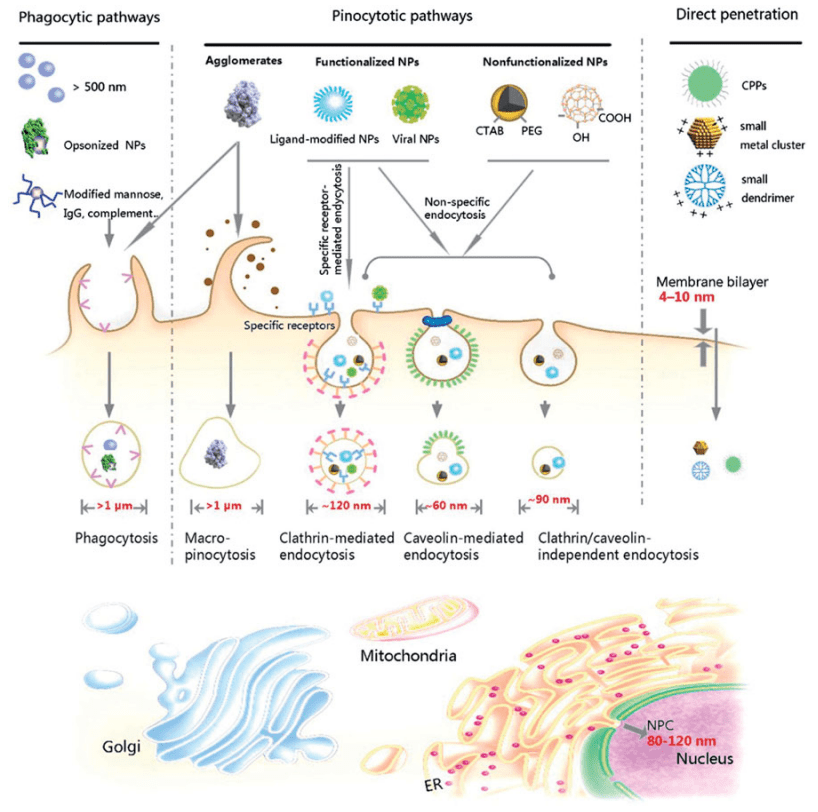
Similar to all polar and charged biomolecules, nanoparticles cannot simply diffuse through the hydrophobic plasma membrane. Nanoparticles employ a highly regulated, energy-requiring form of endocytosis called pinocytosis to enter the cells.
In response to certain stimuli, the cell membrane folds and creates small intracellular vesicles that contain the internalized extracellular liquid and other small molecules—in this case, the nanoparticles. The vesicles subsequently fuse with early endosomes and are then sorted to the lysosomal, recycling, or other trafficking pathways. One method to avoid lysosomal and recycling pathways is to attach pH sensitive targeting molecules via hydrazones to a carrier. Read our article on hydrazone bioconjugation methods for more information.
Based on the cell types involved, the size of the nanoparticles, and the time required, pinocytosis can be subcategorized into four different mechanistically distinct pathways. A summary of each pathway is provided below.
Mechanism 1. Clathrin Mediated Endocytosis
Clathrin-mediated endocytosis occurs in all mammalian cells and is the best understood principal route for cells to obtain nutrients. Internalization of nanoparticles via clathrin-mediated endocytosis occurs in the specialized regions of the plasma membrane containing the triskelion-shaped clathrin protein and AP2.
Activation of these membrane components drives the bending of the membrane, concentrating 100–350 nm-sized nanoparticles into clathrin-coated vesicles (~120 nm) that subsequently disassembles to form an early endosome and onwards to a late endosome that subsequently fuses with a lysosome. As such, clathrin-mediated endocytosis is more suitable for targeted delivery systems for nanoparticles containing cargos not susceptible to lysosomal enzyme degradation (Manzanares and Ceña, 2020; Behzadi et al., 2017; Salatin and Khosroushahi, 2017).
Mechanism 2. Caveolin Mediated Endocytosis
Caveolin-mediated endocytosis involves bulb-shaped membrane invaginations called caveolae (little caves). Upon activation of the receptors—caveolin-3 in muscle cells and caveolin-1/-2 in most non-muscle cells—the caveolae detach from the plasma membrane to create a pH-neutral cell compartment called caveosomes (~60 nm).
Compared to clathrin-mediated endocytosis, caveolin-mediated endocytosis requires a longer time and it is more dominant for intracellular delivery of small-sized nanoparticles (20-100 nm). However, it is believed to be more beneficial in enhancing the delivery of nanoparticles carrying enzyme-sensitive drug cargos, as the caveosomes bypass the lysosomal degradation pathway and are delivered directly into the target organelle (ER, nucleus, etc.).
Mechanism 3. Receptor-Mediated Endocytosis
In cells devoid of both clathrin and caveolin, the endocytosis of nanoparticles can be regulated through multiple effectors present on the cell surface, such as Arf6, flotillin, Cdc42, RhoA, etc. Most of these pathways are dynamin-independent and appear to require specific lipid and cholesterol compositions in the cell membrane—although the role of dynamin in some pathways is still being researched (Doherty and McMahon, 2009; Zhang et al., 2016; Sandvig et al., 2018).
Mechanism 4. Macropinocytosis
Macropinocytosis can occur in almost any cells that are deprived of clathrin and caveolin—except for brain microvessel endothelial cells. Unlike other pathways that are initiated by binding/contact of the nanoparticles with the cell surface, macropinocytosis is a non‐specific, actin-driven process in which a large volume of surrounding extracellular fluid and its molecules (>200 nm) are internalized within large (0.5–10 μm), heterogeneous vesicles called macropinosomes that can either fuse with late endosomes, lysosomes or recycle their cargo to the membrane (Lin et al., 2020; Donahue et al., 2019).
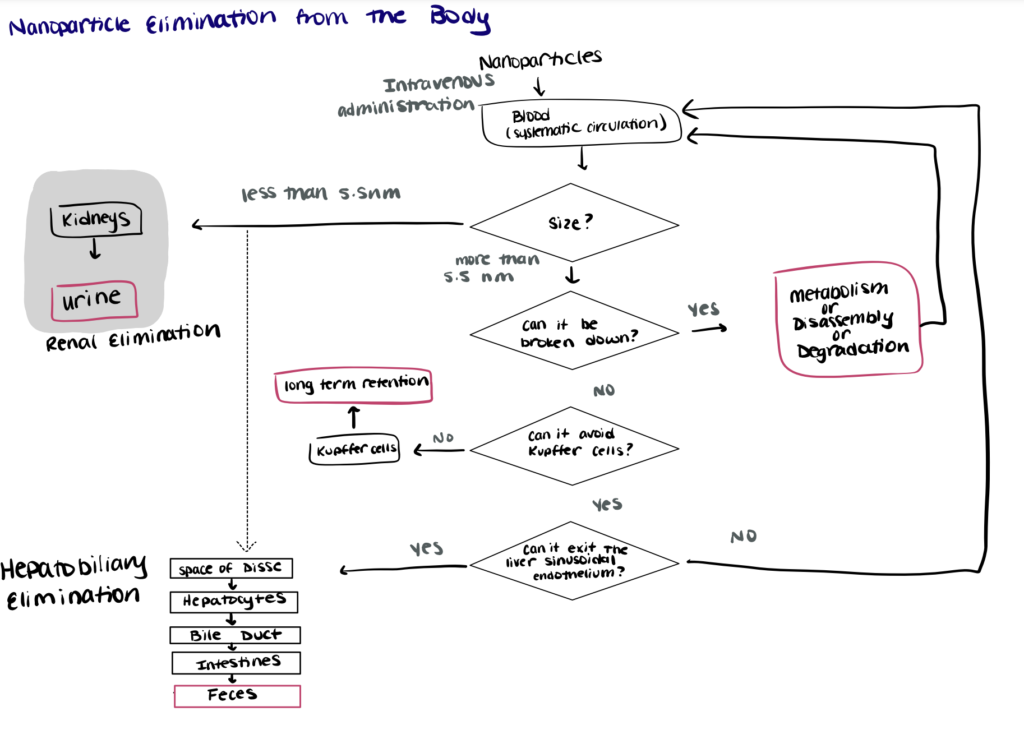
Nanoparticle Clearance from the Body
Nanoparticle clearance from the body is typically carried out by the renal-urine pathway or the hepatobiliary-feces pathway.
Intravenously injected nanoparticles circulate in the blood until they are cleared from the circulation and eliminated from the body following two main pathways, the renal-urine pathway and the hepatobiliary-feces pathway. Most biodegradable nanoparticles with sizes below the threshold required for kidney filtration (~5.5 nm) follow the renal elimination pathway—the preferred route of excretion as it offers optimal clearance characteristics that will minimize toxicity risks. (Liu et al., 2013; Adhipandito et al., 2021). Biodegradable nanoparticles with sizes larger than 5.5 nm can be degraded and/or metabolized to be returned to the systemic circulation and be cleared by the kidneys (Song et al., 2016).
Nonbiodegradable (inorganic) nanoparticles and some biodegradable nanoparticles with sizes larger than 5.5 nm undergo hepatobiliary elimination. Compared to the renal elimination pathway, the principles governing hepatobiliary elimination of nanoparticles are relatively unexplored. However, studies have suggested that the nanoparticles enter the hepatobiliary pathway via transcytosis through liver hepatocytes before they are eventually eliminated in feces. Hepatobiliary elimination of nanoparticles is usually slow and the nanoparticles often accumulate in Kupffer cells—resulting in low passive targeting specificity and long-term toxicity that hinders the translation of such nanoparticles for clinical trials (Poon et al., 2019; Zhang et al., 2016).
Bioconjugation and Cellular Uptake
Nanoparticles are affinity-based biomolecules, in which the endocytic pathway varies depending on the physicochemical characteristics (materials, size, shape, surface chemistry/charge, etc.) of the nanoparticles and the cell type where they were found. Given that many types of nanoparticles possess easily modifiable surface chemistry, for nanoparticles, bioconjugation and cellular uptake are interrelated. It’s possible to create novel NP designs with highly specific architecture to modify their mechanism of uptake, biological function, and prolong their circulation time (Yoo et al., 2010; Salatin and Khosroushahi, 2017).
For nanoparticles to exert their therapeutic effects, they need to be efficiently delivered to target cells, internalized efficiently, and exhibit minimal cell toxicity. However, inefficient cellular delivery due to poor membrane permeability is one of the key factors limiting the high therapeutic efficacy conferred by the therapeutic nanoparticles. Another significant problem associated with the use of nanoparticles includes their short half-life and limited accumulation at the target site, especially for systemic applications of nanoparticles. Most nanoparticles are rapidly removed from blood circulation by the RES organs (Owens and Peppas, 2006), or quickly opsonized and cleared by the macrophages in the liver and spleen (Yoo et al., 2010; Wani et al., 2019).
For the last decade, the spotlight is shown on the versatility and unique properties of polysaccharides to enhance the treatment effects and to reduce side effects of nanoparticles in a highly controlled manner in vitro and in vivo.
Bioconjugation to chitosan, folate, and hyaluronic acid can increase cellular uptake via different mechanisms such as clatherin, receptor-mediated, and caveolin-mediated uptake, respectively.
Method 1. Chitosan-functionalization to increase clathrin-mediated uptake
PLGA is commonly used to develop microparticles and nanoparticles to enhance the solubility/stability of hydrophobic nanoparticles (Lanao et al., 2013). However, the anionic surface of PLGA-nanoparticles causes low cell compatibility and internalization (Wang et al., 2021; Wang et al., 2013).
Chitosan (CS), a positively charged carbohydrate chain derived from Chitin from crustaceans, increases the internalization of nanoparticles. CS functionalization changes the surface charge of PLGA to positive to further improve their uptake efficiency, especially with the negatively charged clathrin proteins on cell surfaces (Jayakumar et al., 2007; Wang et al., 2013; Makadia and Siegel, 2011). Similarly, positively charged peptides (also known as cell penetrating peptides or CPPs) are an excellent way to increase cellular uptake of negatively charged molecules. We’ve discussed peptide bioconjugation in more detail in another article.
Here’s a brief method to conjugate CS and PLGA to increase the delivery potential of nanoparticles via clathrin-mediated uptake. For a full protocol, read this article.
If your carbohydrate has readily available amines or carboxyl groups, you can use these kits to attach it to other biomolecules like proteins, antibodies, and polymers.
Step 1. Preparation Of PLGA-Nanoparticles
In this study, the authors used uncapped PLGA with free carboxyl (COOH) termini for better drug encapsulation properties and conjugation of ligands. The PLGA-nanoparticles were prepared using the physical adsorption method in a modified aqueous drug solution/organic phase/external phase (5-FU/PLGA-DCM/surfactant PVA) emulsion. Following solvent evaporation, the PLGA-nanoparticles were then free-dried and preserved for further evaluation.
Step 2. Bioconjugation Of CS To PLGA-Nanoparticles
The freeze-dried PLGA-nanoparticles were then dissolved in a PBS solution containing NHS and EDC to activate the carboxyl group of PLGA. Different concentrations of chitosan were then added into the solution containing the activated PLGA-nanoparticles and then incubated for 24 h. The excess EDC, NHS, and unreacted chitosan were eliminated by centrifugation.
Step 3. Characterization Of CA-PLGA-Nanoparticles
The results from the particle size, zeta potential, FTIR, and XPS analyses conducted following chitosan treatment all indicated that the amine groups in the chitosan structure have successfully conjugated to the surfaces of the PLGA-nanoparticles.
The CS-PLGA-nanoparticles were shown to exhibit a positive charge surface and the zeta potential dramatically decreased as pH value increased due to the presence of amine groups on its surfaces. The positive charge and increased hydrophilic property also resulted in prolonged and higher cumulative drug release profiles compared to the unmodified PLGA-nanoparticles in vitro.
Method 2. Folate Functionalization To Increase Folate-Receptor Mediated Uptake
Recently, nanoparticles are becoming more popular because they can be used to develop targeted drug delivery vehicles for diseases like cancers. Among different polymeric materials available, alginate provides a great opportunity for bioconjugation and cellular uptake of drugs due to its specific structure and outstanding biological properties (Maeda et al., 2000; Wang et al., 2015).
In a recent study, a team of authors developed a novel phytosterol-alginate (PA) nanoparticles design functionalized with folate to target drugs to folate receptors-overexpressing cancer cells and avoid cytotoxicity to normal tissues. The full protocol can be found here.
Step 1. Preparation Of Nanoparticles
In this study, self-assembled nanoparticles were prepared using the probe sonication method. After the folate-phytosterol-alginate (FPA) was dispersed in distilled water, the solution was sonicated three times until the desired size and shape of the nanoparticles had been attained.
Step 2. Characterization of FPA-nanoparticles
The physicochemical properties of the FPA-nanoparticles were characterized by NMR, TEM, DLS, ELS, and fluorescence spectroscopy. The results indicated that the phytosterols and folate were successfully conjugated to the alginate.
Read our article, bioconjugation characterization for more information regarding characterization via spectroscopy or DLS methods.
Step 3. In vitro Drug Release Profiles
In order to further evaluate the role of folate in the cellular uptake of the FPA-nanoparticles, DOX was physically entrapped in the nanoparticles by the dialysis method. The results indicated the DOX-FPA-nanoparticles showed excellent drug-loading and releasing efficiency, and the release of DOX was pH-sensitive and occurs more rapidly in an acidic environment normally observed in cancer cells—thereby reducing cytotoxicity in normal tissues.
Step 4. Assessment Of Cellular Uptake Mechanisms
Results from the cytotoxicity, folate competition assays, and CLSM analysis all suggested that the DOX-FPA-nanoparticles were taken up by cells through the folate-receptor-mediated endocytosis mechanism. Upon internalization, the DOX-FPA-nanoparticles were shown to escape the endolysosomal pathway and enter the cytoplasm directly. For an alternate method for receptor-mediated uptake consider biotinylation. We’ve written an article on biotinylation and cellular uptake here.
Method 3. Hyaluronic Acid Bioconjugation Increases Caveolin-Mediated Uptake
In recent years, the use of hyaluronic acid (HA) to develop nanoparticles with superior characteristics has been widely studied. HA-functionalized nanoparticles have been widely utilized for targeted delivery to malignant tumor cells due to their high affinity towards the CD44 and RHAMM receptors. Additionally, the use of HA-based nanoparticles has also been stimulated by the abundance of readily-modified carboxyl and hydroxyl groups on HA surfaces (Saltatin and Khosroushahi, 2017, Wang et al., 2013).
Read on to see how a team of authors developed an innovative formulation of mucoadhesive nanoparticles consisting of oligomers of chitosan (CSO) and HA for gene delivery to the ocular surface mediated by hyaluronan receptors through the caveolin-dependent endocytic pathway. For a full protocol, read this article.
Step 1. Preparation of Hyaluronic Acid-Chitosan Nanoparticles
HA-CSO nanoparticles were made from fluoresceinamine labeled HA (fl-HA) and CSO by using a slightly modified ionotropic gelation technique. The FL-HA was first mixed with TPP crosslinker and added over CSO solution under magnetic stirring. You might also consider radiotracers for tracking your cellular uptake pathways, consider our article on bifunctional chelating agents to learn more about this approach.
The physicochemical and morphological properties of the nanoparticles were then characterized. The HA-CSO nanoparticles were then loaded with a model pDNA encoding secreted alkaline phosphatase (SEAP) by incorporating the pDNA in the HA/TPP.
If your carbohydrate has readily available amines or carboxyl groups, you can use these kits to attach it to other biomolecules like proteins, antibodies, and polymers.
Step 2. Uptake and Trafficking Experiment
In this study, the authors used cornea-derived HCE cells and conjunctiva-derived IOBA-NHC cells to determine the kind of endocytic pathway implicated in the HA-CSO-NP uptake. The cells were treated with chlorpromazine (CPZ) and filipin, the clathrin- and caveolin-dependent endocytosis inhibitors, respectively. As the uptake was significantly inhibited by filipin, the authors concluded that the HA-CSO NP uptake is mediated mainly by caveolin-dependent endocytosis through the CD44 receptor.
Further confirmation using fluorescence microscopy and intracellular NP-associated fluorescence quantification were consistent with the previous result. The degradation pathway of nanoparticles following internalization also showed no evidence of NP degradation by lysosomes.
