One popular form of bioconjugation is azide bioconjugation, in which azides are frequently conjugated with alkynes. Since neither azides nor alkynes are found in cells or biological systems, the reactions are bioorthogonal, meaning they won’t interfere with native processes inside of a cell (Li & Zhang, 2016). One of the most powerful applications of bioconjugation is the copper-catalyzed azide alkyne cycloaddition (CuAAC). However, researchers are now turning to alternative azide bioconjugation reactions that don’t use copper as a catalyst; this is important because copper is cytotoxic and can potentially influence the system studied (Nwe & Brechbiel, 2009).
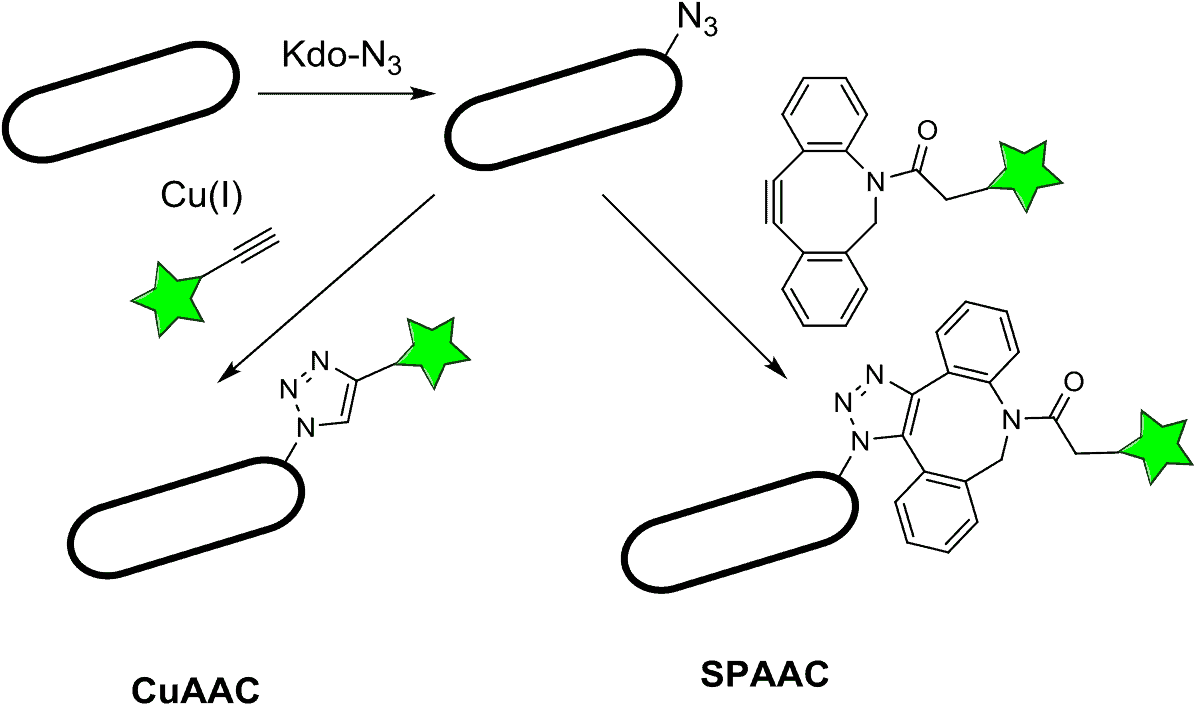
Common azide bioconjugation reactions include copper-catalyzed azide alkyne cycloaddition, or CuAAC, and strain-promoted azide alkyne cycloaddition, or SPAAC.
Related articles:
- Azide bioconjugation reactions are commonly utilized for bioconjugation to nanoparticles
- Enzymat-catalyzed bioconjugation can be utilized to add azide groups onto proteins
What Is Azide Bioconjugation Chemistry?
Bioconjugation is a technique in which a biomolecule is covalently bound to another molecule via a linker. It is often used to attach probes to proteins and other biomolecules to help with molecular visualization and detection in assays like Western blotting and FRET. The linker, or conjugation agent, will attach to each joining molecule by reacting with a specific functional group, such as a thiol or azido group; sometimes secondary activating agents are used to induce reactivity in the target functional group (Hermansen, 2013).
If you are interested in learning more about western blotting, consider our articles, western blot exposure time and detection or how to choose western blot loading amounts.
Azide bioconjugation chemistry is a subset of click chemistry that uses azides and other functional groups such as alkynes.
Kolb, Finn, and Sharpless first coined the term “click chemistry” in 2001, originally referring to a wide selection of useful bioconjugation reactions (Kolb et al., 2001). While click chemistry still refers to that wide array of reactions, azide bioconjugation is among the most powerful and utilized among them (Li & Zhang, 2016; Nwe & Brechbiel, 2009). The CuAAC reaction is even commonly referred to as the “click reaction” (Avti et al., 2013).
In 1963, Huisgen discovered the first reactions of uncatalyzed azide alkyne cycloaddition. However, it required such high pressures that it was impractical. It was only after Kolb et al. catalyzed the reaction with copper that it grew in use and popularity. In recent years, more concerted efforts have grown to find non-copper-based catalysts to combat copper’s cytotoxic effect on study systems (Nwe & Brechbiel, 2009).
Copper-free iterations of the click reactions are called Strain-Promoted Azide Alkyne Cycloaddition (SPAAC). These reactions use reagents called cyclooctynes such as dibenzocyclooctynes (DBCO) and difluorocyclooctynes (DIFO) (“Cu – Free Click chemistry”; Nwe & Brechbiel, 2009).
One of the key features of CuAAC and SPAAC are that they are bioorthogonal. This means that they can be performed in the presence of other functional groups without any side reactions — this is a common problem with bioconjugation reactions (ie. they are very specific).
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Copper-Catalyzed Azide Alkyne Cycloaddition (CuAAC)
In copper-catalyzed azide alkyne cycloaddition, or CuAAC, copper(I) catalyzes a reaction between alkyne and azide functional groups, resulting in 1,4-substituted triazoles.
In the original Huisgen reaction, products were much less regiospecific, as a mixture of 1,4- and 1,5- substituted triazoles were formed. The lack of specificity in the original reaction, as well as the high requisite temperatures and pressures, are part of why it was not useful at the time. The copper-catalyzed iteration of the reaction also allowed for a wider range of acceptable pHs, solvents, and reagent structures (Pickens et al., 2017). A proposed detailed mechanism of the reaction is shown below. There is debate over the reaction mechanism, as some think multiple copper ions are needed (Li & Zhang, 2016).
Applications of Azide Bioconjugation Using CuAAC
Some applications for azide bioconjugation reactions using copper-catalyzed azide alkyne cycloaddition (CuAAC) include the creation of contrast agents for MRI, detection of cell cycle phases using fluorescent probes, and visualization of viral proteins.
Related articles:
- Palladium catalysts for bioconjugation enable site-specific modification of cysteine residues
- Bioconjugation with NHS esters enables you to react specifically with amine containing amino acids like lysines
Application 1. Creation of A Contrast Agent For MRI
Bryson (2008) et al. used a variety of synthesis strategies including the click reaction to synthesize a new contrast agent for MRI scanners. The click reaction was ideal because it worked despite the potential steric hindrance from seven chelates bound to the central cluster.
Application 2. Detection of Cell Cycle Phase
Cappella (2008) et al. used the click reaction to bioconjugate 5-ethynyl-2’-deoxyuridine (EdU) that was incorporated into the DNA of a cell with 5-bromo-2’-deoxyuridine (BrdU) azide. Anti-BrdU monoclonal antibodies with a fluorescent probe attached were then used to quantify amounts of DNA to determine cells’ placements in the cell cycle.
Application 3. Visualizing Viral Structural Proteins
Serwa (2019) et al. introduced L-azidohomoalanine (AHA) to cells before infecting them with the herpes simplex virus (HSV-1). Upon separating viral lysates from infected cells, they used CuAAC to bioconjugate the AHA to Alkynyl-TAMRA-Biotin (YnTB), which contains an alkyne group and a fluorescent probe. Results were then analyzed with SDS-Page.
A Brief Protocol Involving CuAAC Bioconjugation
In the following steps, we briefly describe a step-by-step method involving CuAAC bioconjugation chemistry for the application of creating protein dendrimers for clinical and biochemical uses. You can find the full research article here.
Step 1. Synthesize The Scaffolding Protein
Synthesize per-6-deoxy-6-azido-β-cyclodextrin (β-CD(N3)7) from β-cyclodextrin. To do so, first treat β-CD with PH3P and I2, then react it with NaN3.
Step 2. Synthesize The Peptide
Synthesize the peptide, Gly-Gly-D-Pra, using 9-Fluromethoxycarbonyl (Fmoc) chemistry in standard solid phase peptide synthesis protocols.
Step 3. Express And Purify Proteins
Express and purify sortase A from S. aureus, a fragment of the Pneumoccocal surface protein (PspA) from S. pneumoniae, and a pilus protein RrgB from S. pneumonia in E. coli expression systems.
Step 4. Label The Proteins And Peptides With Alkynes
Label the previously expressed proteins, PspA and RrgB, and the previously synthesized peptide, Gly-Gly-D-Pra, with alkynes via sortase-mediated ligation. To do so, incubate the proteins and peptides with SrtA and Buffer S, then run them through nickel column chromatography. The sortase protein will catalyze the reaction.
Step 5. Perform CUAAC Using Peptides
Conjugate (β-CD(N3)7) with Gly-Gly-D-Pra using CuAAC in a solution of sodium ascorbate and copper (II) sulfate. The peptide will now have an alkyne group which can react with the azide groups on the scaffolding protein. The sodium ascorbate will reduce the copper to copper (I) so that it can react.
Step 6. Perform Cuaac Using Proteins
Conjugate (β-CD(N3)7) with the alkyne-labeled PspA and RrgB proteins using the same process as described in Step 5 (Singh, 2019).
Strain-Promoted Azide Alkyne Cycloaddition (SPAAC)
Strain promoted azide alkyne cycloaddition or SPAAC uses cyclooctynes like DBCO and DIFO for click chemistry. In this method, the ring strain on the cyclooctyne is relieved during the reaction with the azide. Hence, the reaction can take place without a copper catalyst.
Copper-free click reactions, also known as SPAAC, work using cyclooctynes like DBCO and DIFO. The alkyne group is found on the cyclooctyne, and the reaction is driven by large amounts of ring strain in the cyclooctyne; the reaction helps to relieve the strain. This reaction solves the cytotoxicity problem of the CuAAC, and also proceeds readily at reasonable temperatures and pressures (“Sigmaaldrich”; “Cu – Free Click Chemistry”)
Applications of Azide Bioconjugation Chemistry Using SPAAC
Applications of azide bioconjugation chemistry using strain-promoted azide alkyne cycloaddition (SPAAC) include conjugation of DNA to a flow cell surface, conjugation of exosomes for delivery into cells, and detection of bacteria at low concentrations.
Polymers can be conjugated to proteins and antibodies using a range of functional groups such as amines, carboxyls, or thiols. Explore conjugation kits for polymers, proteins, and antibodies, here.
Application 1. Conjugation Of Dna To A Flow Cell Surface
Eeftens (2015) et al. first ligated DBCO to DNA. They then attached azides to the coverslip with PEG linkers. This put all of the components in place for copper-free click chemistry and allowed them to click the DNA onto the coverslip. The novel strategy creates a complex that withstands large amounts of strain and can be used to study single-molecule DNA-protein complex experiments. You can learn more about PEGylation and crosslinking using bifunctional crosslinkers here.
Eeftens et al. used SPAAC to conjugate DNA to a flow cell surface. Image source: BMC Biophysics.
Application 2. Conjugation To Exosomes For Delivery Into Cells
Wang (2015) et al. used copper-free azide bioconjugation chemistry to find a way to attach small molecules to modulate proteins in exosomes. They first labeled exosome proteins with azides, then used the click reaction to attach it to cargo-bound DBCO, which can then be taken into the cell. This toolkit is easily customizable and provides the framework for a large array of experiments, as different cargo molecules can be easily delivered to cells.
Application 3. Detection Of Bacteria At Low Concentrations
Fugier (2015) et al. incorporated azides in the LPS of E. coli which are then conjugated with sulfo-DBCO-biotin. Bacteria could then be detected with fluorescent antibodies or isolated via magnetic beads. This provides a novel potential enrichment technique to help detect bacteria at low concentrations.
A Brief Protocol Involving SPAAC Bioconjugation
In the following steps, we briefly describe a step-by-step method involving SPAAC for the application of creating a shorter immuno-PCR method. You can find the full research article here.
Step 1. Attach Azide Groups To Antibodies
Add MA2 and BE5 antibodies and pentynoic acid sulfotetrafluorophenyl ester (STP-N3) into a sodium bicarbonate solution to add azide groups to the antibodies.
Step 2. Attach DBCO To Oligonucleotides
Add ODN Ip3-am oligonucleotides to a buffer of NaHCO3 and DMSO. Then add DBCO-PEG4-NHS, which will react with the oligonucleotides and attach DBCO to them.
Step 3. Perform SPAAC With Antibodies And Oligonucleotides
React the modified antibodies with the modified oligonucleotides at room temperature in a 1:4 ratio of active groups. The azide groups on the modified antibodies react with the alkyne groups on the DBCO portion of the modified oligonucleotides in a copper-free click reaction, creating bioconjugates.
Step 4. Analyze Results With Immuno-PCR
Use Immuno-PCR with the new bioconjugates to test whether the process is viable (Maerle, 2019).
