Protein bioconjugation has become an ever expanding topic of interest in the biomedical field. The cysteine residue is characterized by its sulfhydryl group, giving it strong nucleophilicity. The low abundance of cysteines in proteins allows for highly selective functionalization of proteins. We’ll discuss bioconjugation of maleimide and cysteine in this article.
Bioconjugation of maleimides involves a Michael addition reaction between the maleimide (acceptor) and thiolate (donor). Maleimide-thiol chemistry can be utilized to react with cysteine groups on proteins for applications in drug delivery, bioimaging, and biosensing.
Folded proteins can be difficult to functionalize due to their complex structure and sensitivity to pH and temperature changes. To combat these challenges, bioconjugation of the cysteine residues present in folded proteins has become a popular subject. So, now that we know this is an important topic, let’s get into it!
Related articles:
- Using maleimide chemistry is a great way to introduce cysteine containing proteins onto surface. You can read more about bioconjugation to surfaces in our other article.
- A common strategy is to use maleimide chemistry for C-terminal bioconjugation. Read more in our related article.
Maleimide Thiol Reaction Mechanism – Michael Addition
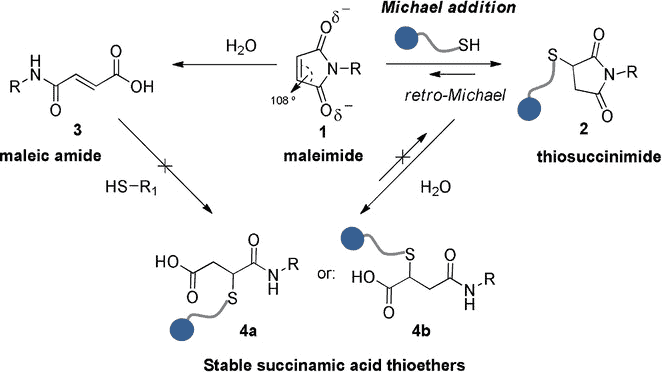
The maleimide thiol reaction mechanism involves a Michael addition reaction where the maleimide acts as the Michael acceptor while the thiolate is the Michael donor.
Depending on how strong a base the nucleophile is, it will undergo either direct addition or conjugate addition. The thiolate acts as a weak base allowing for conjugate addition onto the maleimide forming a thiosuccinimide complex, shown in step 2 below.
For unmodified maleimide, ring opening occurs via hydrolysis, leading to a stable succinamic acid thioether, as shown in steps 4a and b below. This leads to successful conjugation of the cysteine residue. Alternatively the maleimide can undergo hydrolysis to form malic amides as shown in step 3 below, followed by thiosuccinimide hydrolysis. This leads to the same outcome. Note that, since cysteines are very rarely on the N-terminus, so for N-terminus bioconjugation of proteins, you’ll need an alternative method.
Trying to attach molecules together? You can explore conjugation kits to help you attach biomolecules together quickly and repeatably here.
Maleimide Hydrolysis
Although Michael addition reactions lead to selective bioconjugations, there are often problems with instability; thus, making applications in areas such as drug delivery difficult.
Maleimide hydrolysis is utilized to improve the stability of maleimide-thiol conjugates by forcing the thiosuccinimide adducts to undergo ring opening hydrolysis.
By increasing the temperature and alkalinity of these reactions it is possible to improve reaction selectivity to favor thiosuccinimide hydrolysis and thus ring opening of the maleimide. However, as defined in an article by Barradas et al, for significant improvement, this requires pH’s in the range of 10 to 14, which can lead to the protein denaturation. To combat this challenge many researchers have begun investigating new maleimide derivatives to facilitate ring opening reactions.
Maleimide Derivatives and Their Pros/Cons
Maleimide derivatives have been extensively researched and developed over the past several years and many new complexes have been produced to help improve bioconjugate stability.
Maleimide derivatives include N-Alkyl, N-Aryl, and beta amino maleimides. Reactions with N-alkyl maleimides are fast but conjugates tend to destabilize quickly as well. N-aryl maleimides produce stable conjugates but the reaction yields tend to be lower. Beta-amino maleimides exhibit fast kinetics as well as generate stable conjugates.
There are many others and each one has its own advantages and disadvantages, but for brevity’s sake only the aforementioned will be discussed here.
1. N-Alkyl Maleimide
N-Alkyl Maleimides are maleimide derivatives characterized by an alkyl group bound to the amine, often produced using the mitsunobu method.
These molecules exhibit fast reactivity towards thiolates under a wide temperature and pH range however the final conjugates can often undergo retro michael reactions and do not easily undergo hydrolysis making resulting conjugates unstable (from Walker et al).
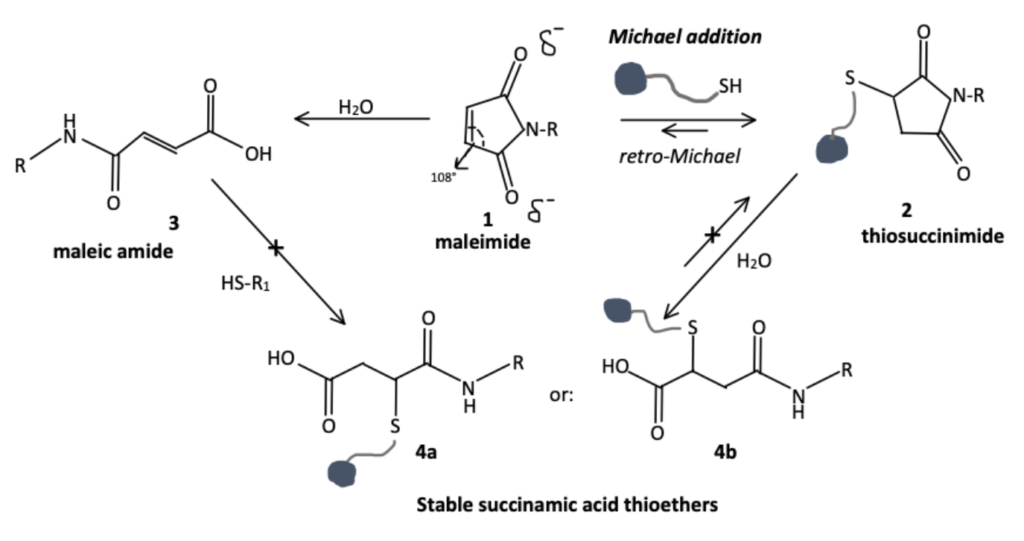
After thiosuccinimide is formed, the lone pair of the nitrogen can delocalize between the nitrogen and the carbonyl groups to give nitrogen a positive charge and to allow for nucleophilic attack by water. This attraction, however, is weak and must be improved by increasing both temperature and pH which may lead to unfavorable conditions for many biomolecules. This has led researchers to investigate modification of the maleimide to improve electron delocalization, and thus hydrolysis.
2. N-Aryl Maleimide
One system investigated to improve electron delocalization is the use of aryl groups. Incorporation of an aromatic ring to form the N-Aryl maleimide improves hydrolysis rates without the need for increased temperature or pH.
The incorporation of an aromatic ring causes resonance delocalization through conjugated pi system resonance (see image below) reducing electron density and making the amine of the thiosuccinimide conjugate more susceptible to hydrolysis.
One study done by Christie et al showed great results for increased rates of hydrolysis and improved stability, however when the system was exposed to a buffer (pH 7.4, 22 ºC, 1 hr) they saw a decrease in conjugation yield of 30-50%.
3. Beta Amino Maleimide
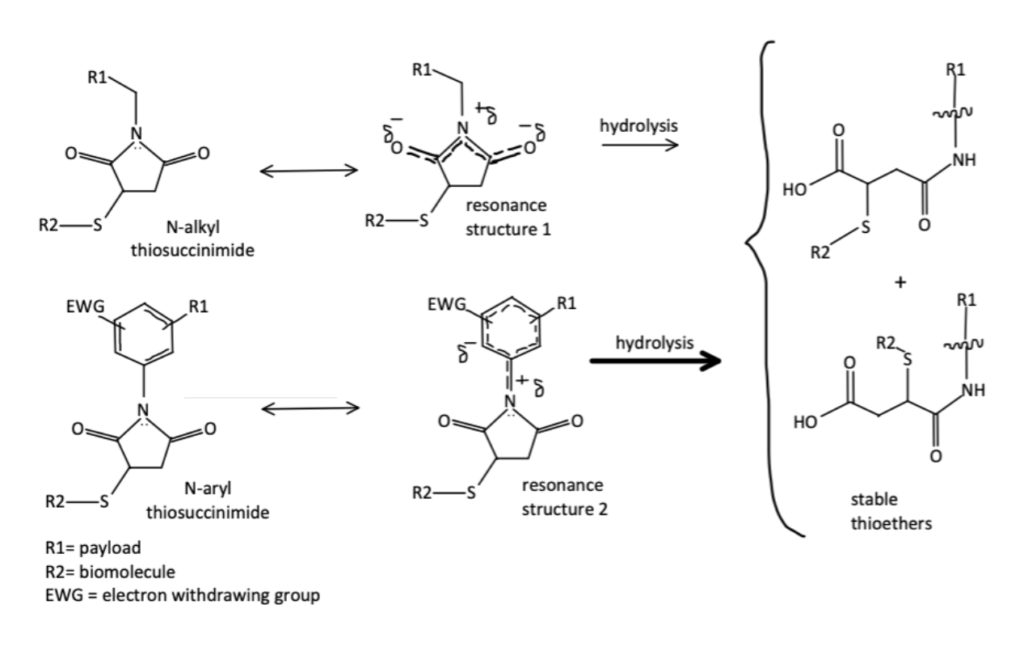
Beta-amino maleimides are formed by attaching an adjacent basic amino group to N-alkyl maleimides.
The addition of the amino group helps to encourage intramolecularly catalyzed hydrolysis reactions which improves the rate of ring opening reactions. Using beta-amino maleimides researchers were able to not only improve rates of hydrolysis and stability, but unlike N-Aryl maleimides, these conjugates remained stable with high conjugation yields in buffered solutions.
One study done by Lyon et al. found that beta amino maleimides underwent rapid ring hydrolysis followed by antibody conjugation leading to superior antitumor activity without an increase in marrow toxicity in mice. These results have led to promising advancements in the fields of bioconjugation and medicine. We’ve discussed other antibody conjugation methods in our related article.
Maleimide Thiol Reaction Protocol / Maleimide Cysteine Reaction
In the following section we provide a general method for bioconjugation of maleimide to a thiol on a cysteine amino acid. Cysteine-maleimide reaction protocols are considered click chemistry and are typically very simple and straightforward. If maleimide chemistry isn’t working sufficiently well for your needs, consider using palladium in bioconjugation of cystine residues, like we have described in our other article.
However, to improve stability a succinimide hydrolysis step is often added, but is optional depending on the linker and application. For more details about this maleimide thiol reaction protocol, read Tumey et al.
Thiols react readily and can be used for conjugation reactions. Papyrus Bio has a range of thiol-based conjugation kits. Explore thiol conjugation kits here.
Step 1. Bioconjugation Of Maleimide And Cysteine Amino Acid
In this step the maleimide is prepared to a 10 mM solution and the prepared cysteine amino acid is added to the solution (8-10 equivalent at 15 mg/ml). The reaction is allowed to incubate for 1-2 hours and then undergoes buffer exchange (pH 7.4) before succinimide hydrolysis.
Step 2. Succinimide Hydrolysis
The maleimide conjugate is buffered into a 50 mM borate buffer (pH 9.2) and subsequently heated to 37 ºC for 24 hours. After the solution cools to room temperature it is buffered into phosphate buffered saline, purified, and then concentrated to 5mg/ml and filtered.
Maleimide Amine Reaction
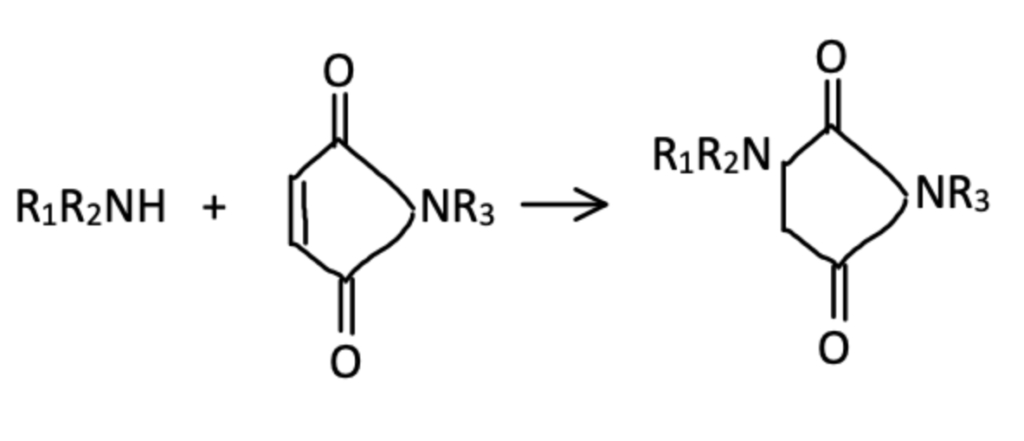
Although cysteine conjugation has been the main topic of interest here, amine groups of proteins can also be targeted and selectively conjugated. In an old paper from 1966, Sharpless and Martin describe that they were able to react maleimide with pyrrolidine, piperidine, benzylamine, and glycylamide. This is a pretty uncommon reaction and will only occur when there are no thiol groups present to react with. These reactions operate best under alkaline conditions, with mild alkalinity being ideal (pH 8.5).
The primary mechanism of conjugation consists of 1,4 addition of the amine to maleimide which ketonizes to the final product (see image below). By increasing temperature and pH it is possible to improve reaction kinetics, however severe conditions may lead to protein denaturing.
The study found that the rate of reaction was highly dependent on the amino compound. The fastest reactants were 3,4-Dehydro-DL-Proline, Piperidine, and L-proline.
These observations grouped with the high number of amine groups available on proteins has made maleimide amine reactions rather undesirable for drug applications. However, they can still be very useful for protein characterization. If you are really worried about side reactions of maleimides, you might consider using enzymatic bioconjugation methods.
Applications for the Bioconjugation of Maleimide
Applications of bioconjugation of maleimide include anticancer treatments, bioimaging and sensing, and detection of post translational protein modifications .
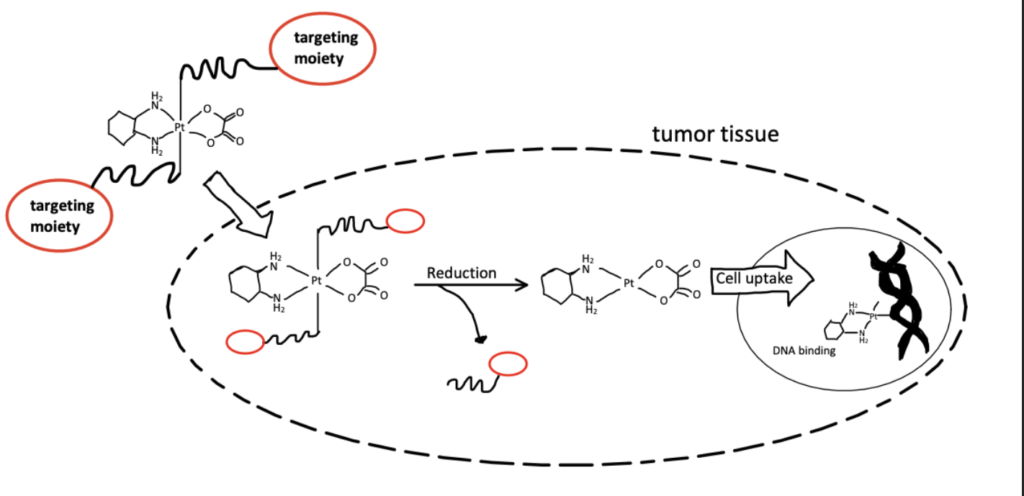
Application 1. Anticancer Treatment
Antibodies have long been used for anticancer drug therapies. Monoclonal antibodies are antibodies that are developed in laboratories for anticancer treatment and are designed to target certain cancer cell receptors. These antibodies are often used to deliver toxic molecules that target and destroy cancer cells such as cisplatin (Pichler et al).
Maleimide conjugate systems are used to attach the drug to monoclonal antibodies for targeted drug delivery. With advancements in maleimide hydrolysis, improved conjugation efficiency, and greater stability, it is possible to improve the quality of drug treatment, prevent illnesses caused by drug therapies, to reduce the costs of production, and thus the overall cost of cancer treatments.
Application 2. Bioimaging and Biosensing
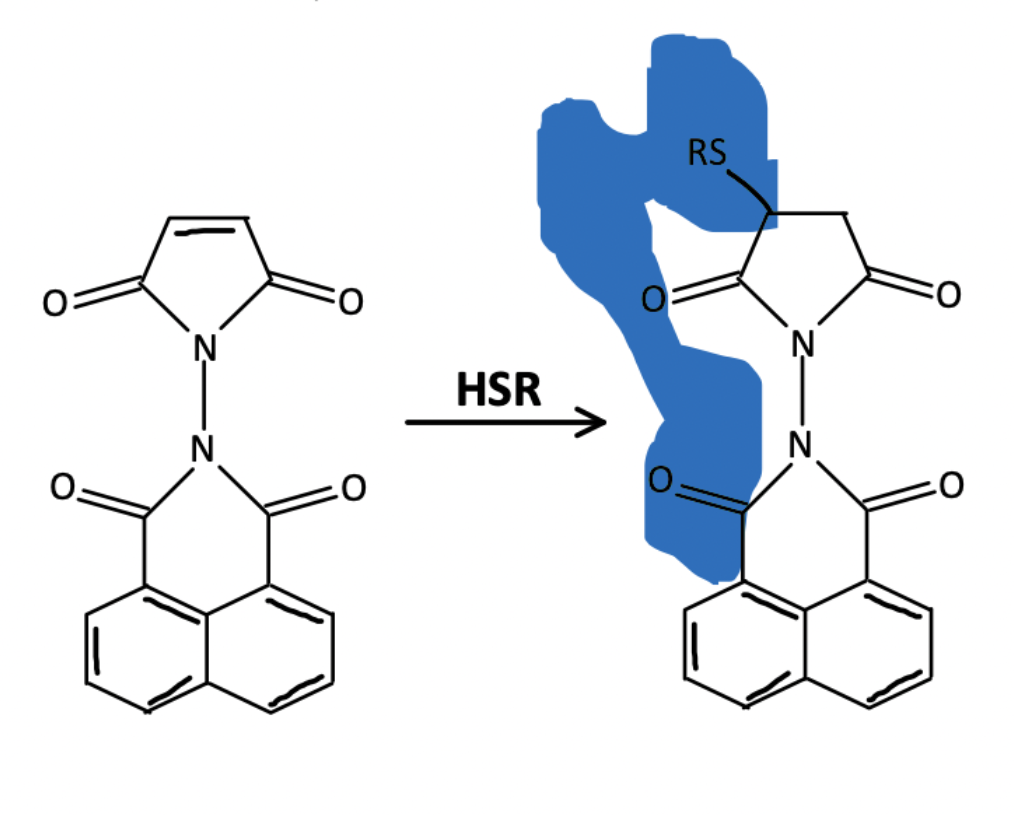
Maleimide conjugates are also often used for bioimaging and sensing applications. Often some type of fluorophore (Cys, Hcy, etc.) is conjugated to the maleimide group which is then incubated with a sample. For example, Qu et al used this method in order to image living cells via confocal fluorescence microscopy. Utilizing FITC labeling, which reacts with amines, is an alternative when you can’t find a maleimide-based linker.
In some cases, Michael addition of the maleimide to thiol groups turns on the fluorescence of dyes, thus allowing for both sensing and imaging to be done via fluorescence detection.
Application 3. Detection of Post Translational Protein Modifications
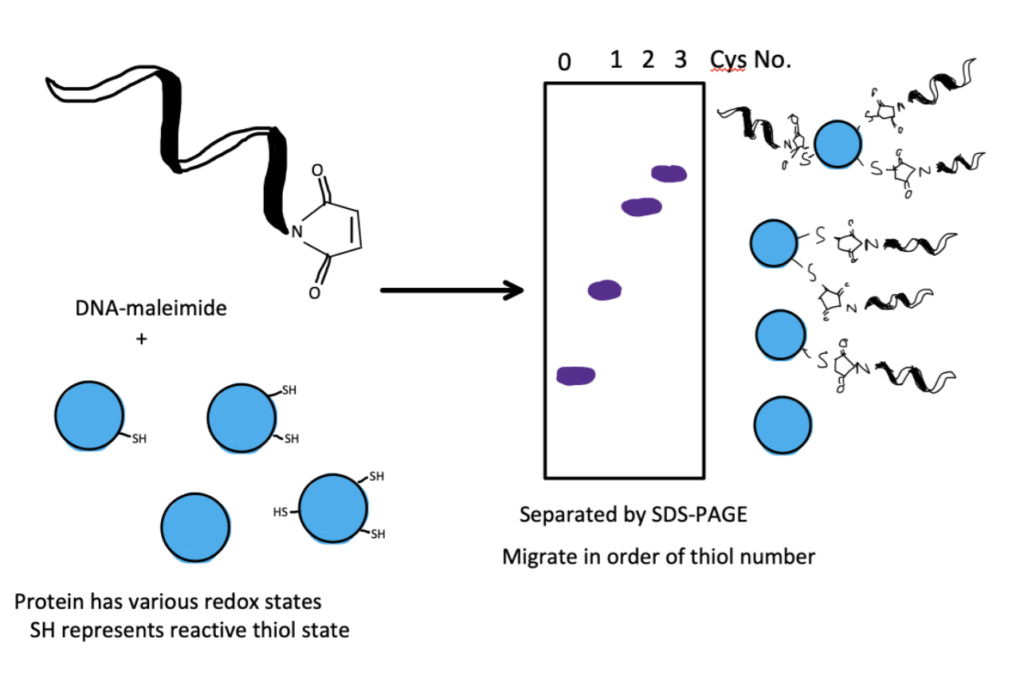
Another application of the bioconjugation of maleimide involves a reaction with the thiolate present on cysteine groups to detect post translational modifications made to DNA and other proteins.
Living cells will often make certain changes to proteins such as glutathionylation, nitrosylation, hyperoxidation, and palmitoylation; commonly known as post-translational modifications. These modifications will often determine how the protein is used in the body, leading to different applications and gene expressions.
During these modifications the thiolate is changed and this can be detected by conjugating DNA-maleimide conjugates to the thiol group and testing via gel electrophoresis. The more thiol groups that are bound by DNA-maleimide complexes, the higher the protein’s electrophoretic mobility. DNA has a natural charge associated with it which propels it through the gel as an electrical field is applied. This can be used as an indication of the status of the thiolate groups on the protein and help researchers understand if there have been modifications (for more information refer to Hara et al.). DNA can also be detected by complexing it with Protamine. Learn more about bioconjugation to protamine and complex formation in our related article.
Want to learn how electrophoresis is used in western blotting? Consider our articles:
western blot exposure time and detection,
