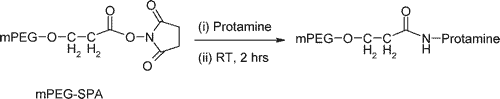
For decades, Protamine has been manufactured primarily as a medication to reverse the effects of heparin during delivery and heart surgery. However, recently it was found to provide membrane-translocating and/or nuclear-localizing activities and there’s increasing literature evidence for the design of novel membrane-translocating peptide-based pharmaceuticals that utilize protamine for delivery. In this article we’ll discuss bioconjugation of protamine and provide protocols and theory behind how and why protamine bioconjugation is utilized.
Protocols for bioconjugation of protamine involve the use of sulfo-SMCC, NHS-ester, and EDC/NHS. To attach protamine to a target molecule, the amine or carboxyl functional groups on protamine are first reacted with a heterobifunctional linker and then attached to the target molecule.
What is Protamine?
Protamine is a low MW polycationic polypeptide 5.5-13.0 kDa) that is highly basic due to its rich arginine (67%) amino acid content. The multiple positive charges of the guanidino groups of arginine within protamine allow it to bind to the negatively charged phosphate groups of DNA to replace 85%–90% of the histones during the late haploid phase of spermatogenesis. Compared to DNA-histone complexes, Protamine-DNA complexes provide the highly compact configuration of chromatin in the nucleus of the sperm for sperm head condensation and DNA stabilization to protect it against degradation during spermatogenesis (Ukogu et al., 2020; Fukushima et al., 2010). Want to learn how to site-specifically conjugate to arginine groups? Read our related article.
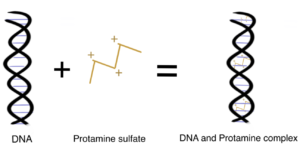
Protamine is a very flexible and well-established pharmaceutical ingredient with a broad range of applications in the biomedical field. Moreover, it has been approved by the U.S. FDA as a carrier to neutralize the anti-clotting effects of heparin and improve the drug release kinetics of injectable insulin formulation Ukogu et al., 2020; Sheng et al., 2016).
Protocols for Bioconjugation of Protamine
Some protocols for bioconjugation of protamine include the sulfo-sMCC-mediated conjugation, the NHS-ester mediated conjugation, and the EDC/NHS-mediated method.
Transglutaminase bioconjugation chemistry can also be used to attach peptides, such as protamine, to proteins.
Method 1. Sulfo-sMCC-Mediated Conjugation of Protamine
Recently, the potential use of siRNA as a therapeutic agent has attracted increasing attention for treating chronic diseases. Cell type-specific delivery of siRNA requires methods that provide efficient transfection/transduction in vivo. Recent studies suggest that bioconjugation of siRNA with cell type-specific affinity ligands, such as therapeutic antibodies, can help improve the specificity of gene silencing in vivo. Additionally, this approach could also enhance the antibody´s efficacy and/or might overcome drug resistance (Lenanova & Poranen, 2018; Bäumer et al., 2016; Dana et al., 2017).
However, developing a strategy to apply siRNAs is a challenge as they are highly charged and short-lived, often failing to be taken up by target cells after systemic application. As such, a team of authors proposed that bioconjugation of protamine to antibodies could further enhance the protection of siRNA against enzymatic digestion.
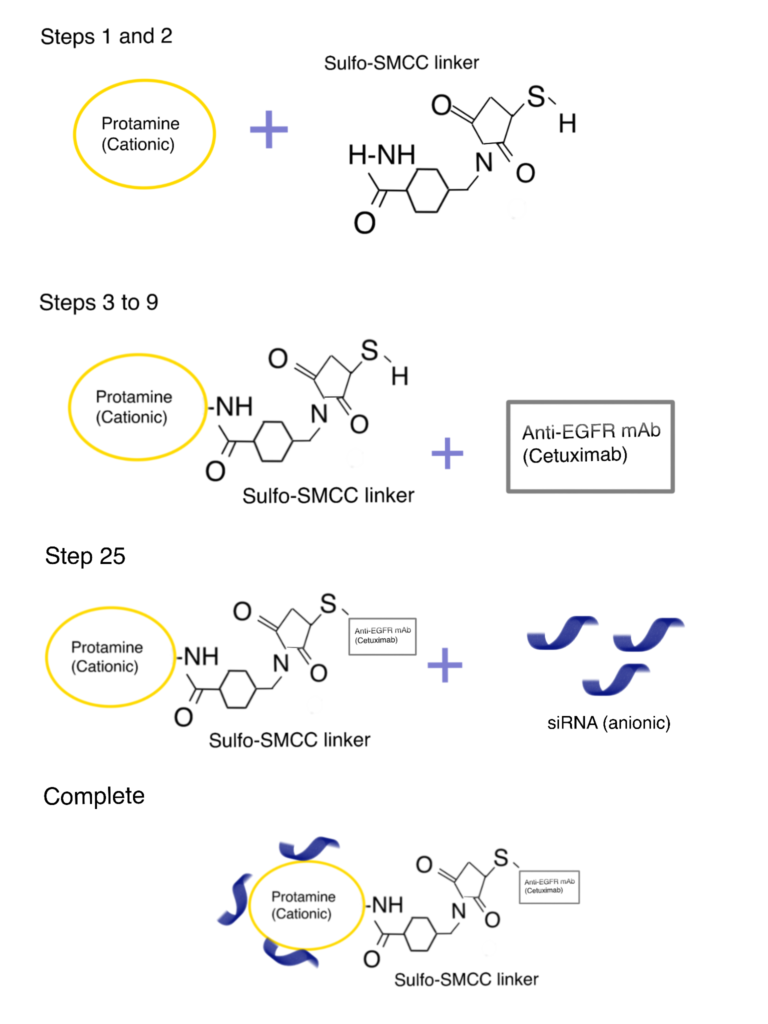
In the following section, we briefly describe a sulfo-SMCC-mediated protamine bioconjugation method utilized by Bäumer et al (2016) to improve the binding and cellular uptake efficiency of antibodies in vivo. For more details, refer to this paper. For alternative antibody bioconjugation methods, read our related article.
Step 1. Antibody–sulfo-SMCC–protamine bioconjugation
First, protamine sulfate (PS) was amino-terminally coupled to the bifunctional crosslinker sulfo-SMCC (room temperature, 2 h, 500 rpm). Next, the sulfo-SMCC-protamine solution was mixed with the desalted antibody solution (overnight at 4°C) to yield the antibody–sulfo-SMCC–protamine bioconjugate by utilizing a cysteine residue within the antibody.
Step 2. Bioconjugation and characterization of antibody–sulfo-SMCC–protamine-siRNA
The siRNA was bound to the protamine within the antibody–sulfo-SMCC–protamine (CSP) complex (CSP) by electrostatic interactions (room temperature, 2 h, 1000 rpm). The resulting complex was shown to efficiently find, bind and internalize into receptor-positive cells in vitro and in vivo.
Method 2. NHS-Ester Mediated Protamine Bioconjugation Method
Cardiopulmonary bypass (CPB) procedures are often associated with massive inflammatory responses that often result in the failure of multiple organs and catastrophic complications during routine cardiac operations. One of the most important factors contributing to this phenomenon is the synergic effect of heparin and protamine, which elicit the activation of the complement system in vivo.
PEGylation of peptides and proteins has been shown to provide important improvements in the pharmacokinetic behavior of various biopharmaceuticals (Veronese & Mero, 2008). It has been proposed that PEG-modified protamine can retain the heparin-neutralization ability and yet diminish the induced complement activation by the formed heparin−protamine complexes—creating a potentially less toxic protamine alternative.
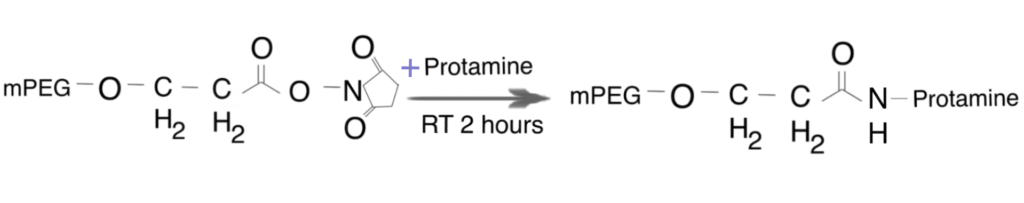
In the following section, we briefly describe an NHS-ester based PEGylation of protamine utilized by Chang et al. (2005). For more details, refer to this paper.
Step 1. Preparation of PEG-protamine bioconjugates
Synthesis of the PEG-protamine bioconjugates was carried out by using the succinimidyl derivative of PEG propionic acid (PEG-SPA). Due to the lack of lysine residues in the protamine sequence, the PEGylation reaction occurred only at the N-terminal proline residue of protamine, a linear PEG-protamine conjugate (PBS, pH 7.5).
Step 2. Characterization of the bioconjugates
Results from studies indicated that the bioconjugates maintain the high reactivity heparin-neutralizing ability and a significantly reduced activity in complement activation following its complexation with heparin, therefore enabling an improved pharmacological activity. In addition, the grafted PEG polymer increases the aqueous solubility profile of the heparin-protamine aggregates, a favorable pharmaceutical property for dosage formulation.
Method 3. EDC/NHS-Mediated Protamine Bioconjugation
In the area of non-viral gene delivery, chitosan has been noted for its ability to protect, condense, and facilitate intracellular transport-release of bound nucleic acid. However, its applications are still limited due to unfavorable physicochemical properties that often leads to premature release of nucleic acids and poor cellular uptake/transfection properties. Depolymerization of chitosan has been shown to overcome the colloidal instability issue and increase its buffering capacity through molecular weight reduction to yield low MW chitosan (LMWC). However, the attempts to depolymerize chitosan may cause the premature release and inadequate protection from nucleases due to lack of affinity for siRNA (Tanasale et al., 2019; Mao et al., 2004; Patil et al., 2016). In this instance, the incorporation of a cationic binding partner into the negatively charged nucleic acid in a chitosan-based polyplex may overcome the binding affinity problem (Patil et al., 2016).
In the following section, we briefly describe an EDC/NHS bioconjugation method utilized by Patil et al. (2016) to incorporate cationic protamine sulfate (PS) into a chitosan-based polyplex for delivery of siRNA therapeutics. For more details, refer to this article.
Step 1. Preparation and characterization of LMWC
First, LMWC was obtained from depolymerization of medium MW chitosan using nitrous acid. The calculated intrinsic viscosity of the LMWC was much lower compared to its native counterpart, indicating improvement in the solubility and colloidal stability properties of the chitosan.
Step 2. Preparation of LMWC–PS bioconjugates
As PS has no primary amines that can be utilized for attaching a sulfhydryl-reactive group, the only carboxyl group in the C-terminus was used for site-specific conjugation. First, the authors activated the C-terminal of PS using EDC and sulfo-NHS (pH 5.5, room temperature, 24 h). These crosslinking agents introduce `zero length’ amide-crosslinks between carboxylic acid groups from glutamic acid residues, and ε-amino groups from lysine residues (Wissink et al., 2001). After completion of the reaction, the pH of the solution was increased to 10 (using 0.1 NaOH) to precipitate the bioconjugates. You can learn more about C-terminal bioconjugation.
Step 3. Characterization of bioconjugates
The results from in vitro and in vivo studies of the bioconjugates indicated protamine imparted positive surface charge at physiologic conditions to facilitate cell interaction and uptake, while the covalent conjugation ensures uptake of sufficient free chitosan as a buffering agent for successful endosomal escape and subsequent gene expression, resulting in improved transfection behavior along with biocompatible nature.
Applications of Protamine Conjugates
Some applications of bioconjugates of protamine include the creation of nanopharmaceuticals, cell-penetrating peptides, and antimicrobial biomaterials.
Application 1. Protamine Based Nanoparticles for shRNA Delivery
Protamine is a very flexible and well-established pharmaceutical ingredient with a broad range of applications, including in the area of nanosized drug delivery research. In this work, protamine NPs were designed by the desolvation method to encapsulate shRNA-expressing plasmid DNA targeting the Bcl-2 gene (shBcl-2) to silence apoptosis-related Bcl-2.
The findings demonstrated that the protamine NPs possessed excellent characterizations and high encapsulation efficiency of the gene. Compared with naked shRNA, protamine NPs could effectively protect shRNA from degradation. Significant improvement of the transfection efficiency and cytotoxicity of in vitro also highlighted the potential of protamine as a nonviral gene carrier. Other examples of the use of protamine in nanopharmaceuticals can be found here.
Nanoparticles are also used in PEG polymer synthesis and subsequent polymer brush bioconjugation. This method of bioconjugation is used in hydrophobic anticancer drug delivery carriers.
Application 2. Utilizing Protamine as a Cell Penetrating Peptide
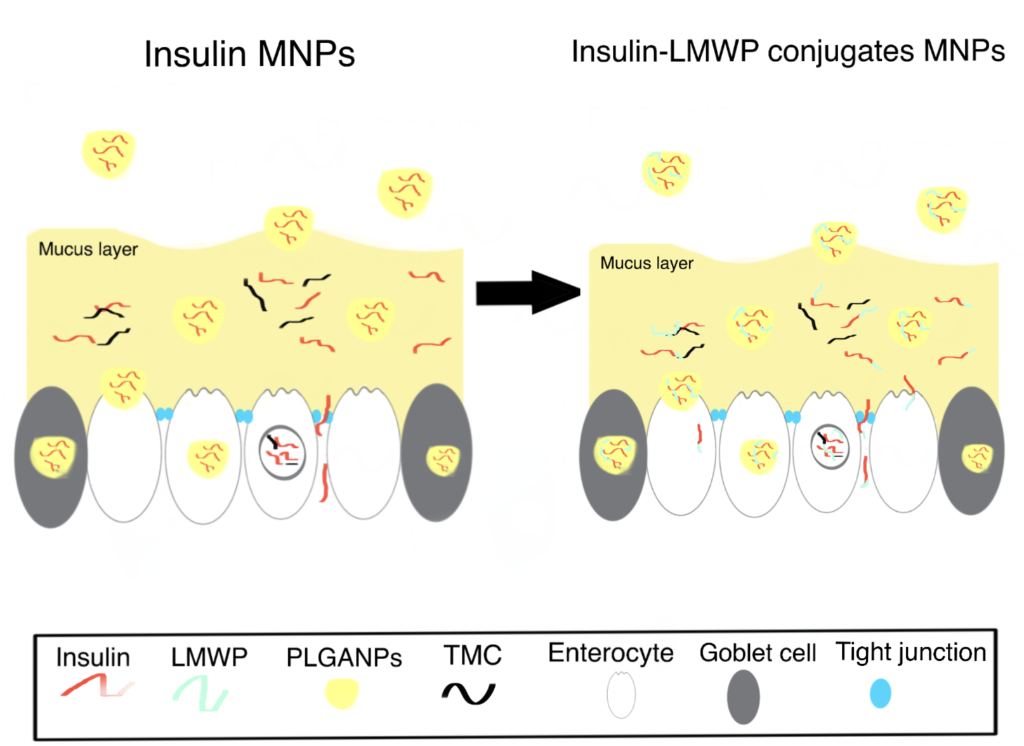
Cell-penetrating peptides (CPPs) have been extensively explored for their potential application in mediating macromolecular drug delivery. Previous studies have demonstrated the capacity of low molecular weight protamine (LMWP) as a potent yet nontoxic CPP capable of translocating protein cargos through the membranes of almost all cell types (Wang et al., 2014).
In this study, Thwala et al. (2018) successfully improved the oral bioavailability of insulin through conjugation with low molecular weight protamine (LMWP), followed by encapsulation in surface-functionalized mucoadhesive NPs (MNPs). As illustrated by the in vitro release results, the insulin-LMWP possessed significantly higher permeability and enhanced retention in the intestinal mucus layer compared to the unconjugated insulin when released from the MNPs. Similar findings suggesting the beneficial effect of protamine-functionalization to enhance the stability and bioavailability of chemotherapeutic agents can be found here.
Another NP, magnetic nanoparticles, have applications as magnetic cell separators; read our article magnetic nanoparticle bioconjugation for more information.
Application 3. Utilizing Protamine for Antimicrobial Biomaterials
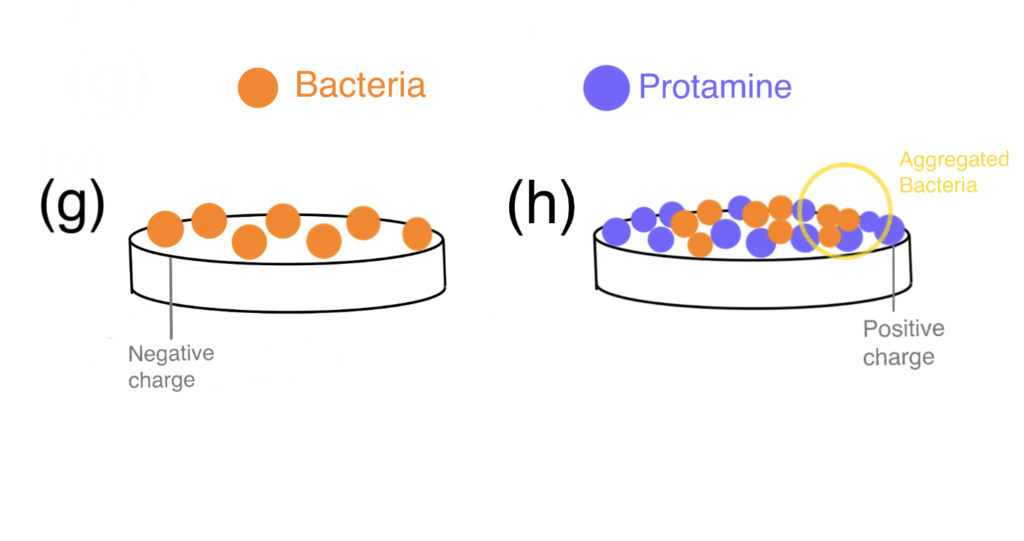
Hydroxyapatite (HAp) has been widely used in the field of bone tissue engineering. However, various bacteria were shown to adhere to the HAp surface and form biofilms—lowering its resorbability and increasing the incidence of implant-related infections (Ribeiro et al., 2012). Against a background of rapidly increasing antibiotic resistance development, surface modifications with antimicrobial peptides/proteins (AMPs) coatings provide scientists a chance at outmaneuvering bacteria. Although protamine shows potent AMP activity in a dose-dependent manner, high dosages of protamine exhibit a cytotoxic effect (Honda et al., 2020).
In this study, Honda et al. (2020) bioconjugation of protamine to HAp (protamine/HAp) was used to create a biomaterial with increased biocompatibility and antimicrobial activity. The authors demonstrated that HAp can adsorb large amounts of protamine with no apparent cytotoxicity in vivo and enable the release of protamine via ion exchange for the long term. With a better release profile, protamine inhibited biofilm formation more effectively and the incidence of implant-associated infections was reduced.
If you are interested in ways to synthesize to antifungal biomaterials, consider our article on bioconjugation using epoxides, where we discuss developing antifungal surface coatings.
