
There are a variety of different accepted routes for utilizing thiol-mediated bioconjugation to create conjugates. These include thiol-maleimide, thiol-para fluoro, thiol-ene, thiol-yne, thiol-vinyl sulfone, thiol–halogeno, thiol–pyridyl disulfide, and thiol–bisulfone reactions.
Thiol-mediated bioconjugation protocols typically involve reactions with maleimides, para-fluoro compounds, alkenes, alkynes, vinyl sulfones, and halogenated isotactic polypropylene. Some applications of thiol-mediated bioconjugation include drug delivery, the creation of glycopolymers for biological applications, and the creation of biologically important monomers like s-glucuronides.
These synthetic routes are used as tools to combine polymers with biomolecules such as carbohydrates, proteins, peptides, DNA, antibodies, or other basic structures in the natural world, which have been found in the last few years (source).
Reactivity of Thiols
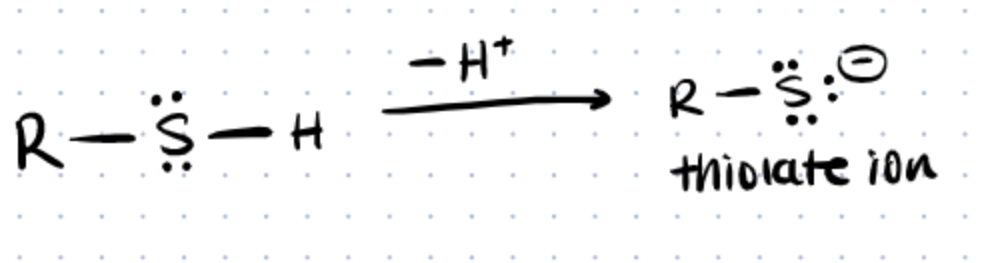
Thiols are any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. Thiols react through a nucleophilic attack on an electrophile by the thiolate. Amines, another common nucleophile, are a composite made up of a basic nitrogen atom with a lone pair (R-NH2). You can read about techniques such as arginine bioconjugation, which utilize amines, in our other article. In addition, you can read more about other alkylation methods on our article about histidine bioconjugation.
Reactivity of thiols can be modified by changing the stability of the thiolate. If the thiolate is stabilized, it reacts faster. For example, reduction of hydroperoxides by the catalytic cysteine of peroxiredoxins is incredibly fast, even compared to free cysteines (source).
Cysteine and glutathione are among the common thiols that can be found in nature. Cysteine is found commonly in keratin as well as other protein molecules, and glutathione is an antioxidant in plants, animals, fungi, some bacteria and in single-cell archaea (source). Along with these, there are also, 2-butanethiol which can be smelled in the unpleasant spray of a skunk that is released as a self defense mechanism, 2-propanethiol (allyl mercaptan) which is a lingering smell on the breath of people that have consumed garlic, and furfurylthiol which adds to the smell of fresh coffee. Thiols such as 2-methyl-2-propanethiol, have such strong odors that they are a great sign and warning for natural gas leaks. (source)
Thiol Conjugation Efficiency
Thiols are a good choice as a reactive group because of their levels of nucleophilicity and their participation in radical-mediated reactions. Because thiols are optimally reactive and naturally abundant, thiol-based chemistry is an obvious choice for protein modification. Bioconjugation of proteins and peptides with polymers is generally performed to protect, make them more soluble in water, or add new functionality onto the proteins. (source)
As with most processes there are challenges involved with thiol conjugation. For example when using thiol-maleimide chemistry to attach drugs to antibodies, it’s possible for maleimides to conjugate to lysine residues. This side-reaction causes an increase in antibody-drug heterogeneity. There is also the factor of maleimide-alkylated peptides being oxidised and hydrolyzed. But, in spite of these negatives, thiol conjugation still remains a viable method of creating bioconjugates. We discuss antibody conjugation methods in more detail in another article.
Thiol Conjugation Techniques
Here we list several different thiol conjugation methods that can be used to develop bioconjugates.
1. Thiol-Maleimide Chemistry
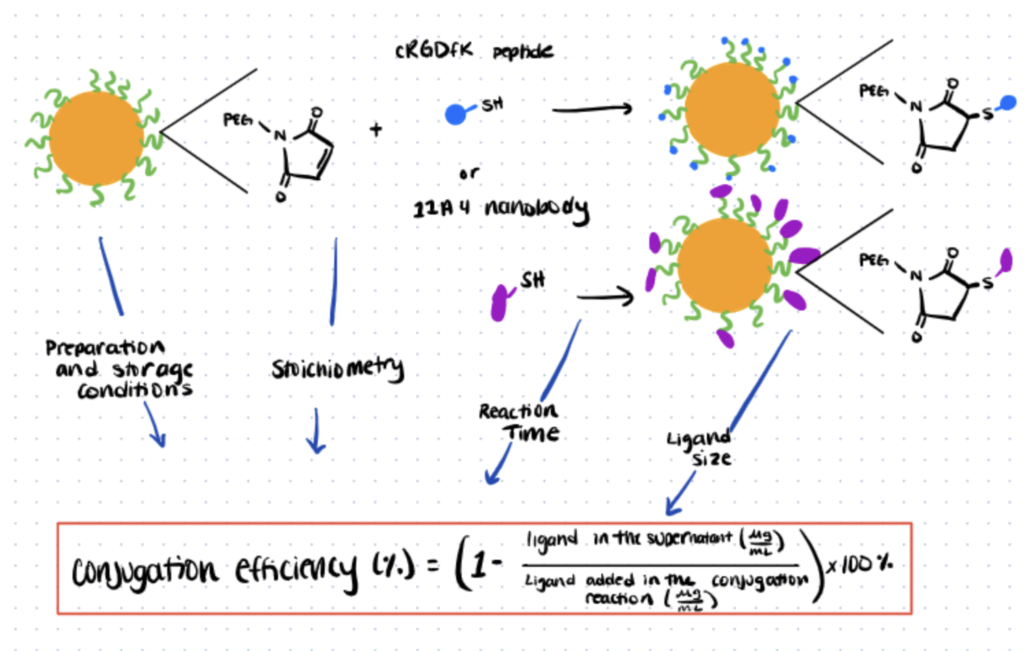
Thiol-maleimide chemistry is extremely common. One up and coming application area is the production of ligand-decorated drug carriers – for example, the creation of poly(lactide-co-glycolide) (PLGA) based nanoparticles (NPs) utilizes thiol-maleimide chemistry.
In this technique below, we utilize the maleimide-thiol click reaction for the conjugation of a peptide and a nanobody that both have a free thiol group. The nanobody is created using PLGA NPs that have been functionalized with maleimide.
The impact that various factors have on the effectiveness of the reaction, such as the method used to prepare nanoparticles, the storage environment, as well as the concentration of the ratio of maleimide to ligand used in the conjugation process, has been assessed and noted in the article that’s linked. For a more detailed protocol, read this article from the Journal of Controlled Release.
Thiols react readily and can be used for conjugation reactions. Papyrus Bio has a range of thiol-based conjugation kits. Explore thiol conjugation kits here.
Step 1. Preparation of NPs
Using a single or double emulsion method with different types and volumes of wetting agent, the NPs are prepared and then stored at around 20 °C before reacting with the peptides..
Step 2. Thiol-Mediated Bioconjugation to Maleimides
The reaction takes place between the maleimide groups within the NPs and the peptides with an optimal reaction at a maleimide to thiol concentration of 2:1, achieving an 84 ± 4% conjugation yield.
It is clear that optimizing this reaction, especially by using the reactants in the correct ratios, can result in noteworthy increases in the efficiency of the conjugation process and mean a substantial reduction in the loss of resources due to waste.
2. Thio–halogeno Chemistry
Thio-halogeno chemistry has been identified as a reliable way to create glycopolymers. Using this type of reaction means having a limited selection of polymerization techniques because halogenides are able to behave as chain transfer agents and can affect initiators and catalysts. A two step process is required to perform this thiol conjugation technique:
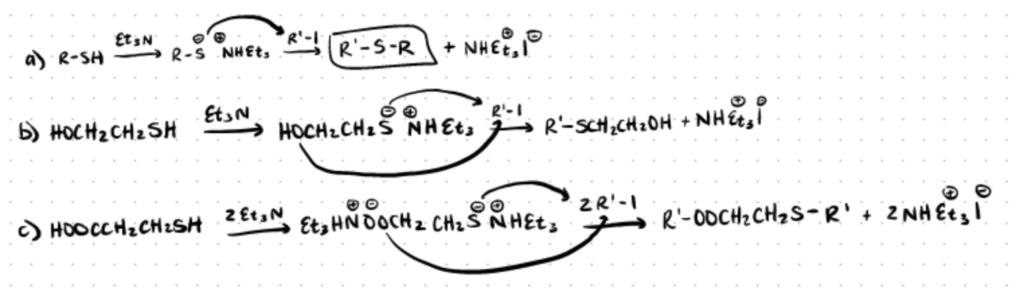
Step 1. Create a Halogenated Polymer
First an iodinated monomer is created. This will later be used to react with the thiolated monomer as shown in reaction A, above.
Step 2. Nucleophilic Substitution and Reaction With Thio-Monomers
Once the halogenated polymers are created, they are reacted with thiolated sugars, with triethylamine (NEt3), K2CO3, or 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) also being present. In many cases, extra thiols are needed for quenching after the reaction is complete.
For equal quantity ratios to be produced, the reaction has a very long reaction time. In spite of this, thiol-halogeno chemistry is still used to prepare glycopolymers because of the creation of a simple thioether bond. For a more detailed protocol, please read this article from ACS Macro Letters.
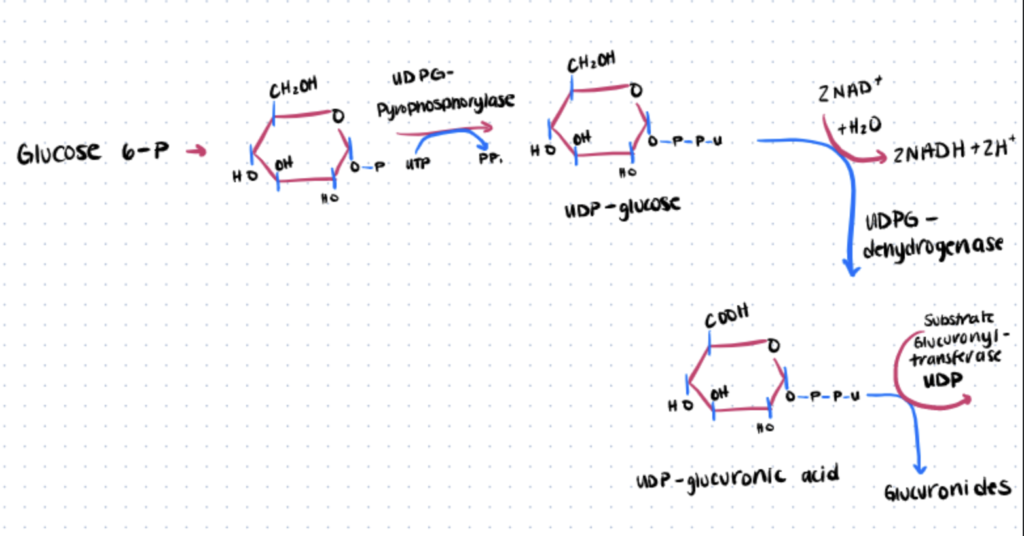
3. Thiol Glucuronic Acid Conjugation
The development of beta-d-glucuronides is catalyzed by UDP-glucuronosyltransferases due to their reaction with UDP-glucuronic acid (UDPGA). It is possible for glucuronic acid to be conjugated to hydroxyl-, thiol-, amino-, hydroxyl amino- and carboxyl-substituents. S-glucuronides are formed from aliphatic thiols, aromatic thiols and dithiocarboxylic acids. Transglutaminase bioconjugation is another common enzymatic method used in bioconjugation reactions.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
Here we provide the process in basic method for creation of S-glucuronides:
Step 1. Obtain Mouse Liver Tissue
The thio-β-d-glucosiduronic acids (thio-β-glucuronides) of o-aminothiophenol, diethyldithiocarbamic acid, p-nitrothiophenol and thiophenol are formed biosynthetically in broken- and complete-cell mouse liver tissue. So, the first step is to obtain the tissue (which expresses the enzyme endogenously).
Step 2. Incubate UDP-Glucuronic acid with Liver Tissue
Next, incubate thio-substituted UDP-Glucuronic acid with the obtained tissue. Note that glucose, glucuronic acid and UDP cannot be used instead. Only UDP-Glucuronic acid can be utilized.
For a more detailed explanation of how s-glucuronides are formed from thiols, read this article, from Advances in Carbohydrate Chemistry and Biochemistry, 2011.
Reversible Bioconjugation using Thiols
Reversible bioconjugation using thiols is possible by using allyl sulfides as a chain-transfer agent along with photo-mediated conjugation.
Reversible bioconjugation using thiols have previously been utilized to conjugate proteins to hydrogels, free them, and then substitute them once the old protein’s activity decreases to keep the overall activity level within the hydrogel high. C-terminus bioconjugation using this technique is a powerful way to create diagnostics and activated protein surfaces.
An allyl sulfide chain-transfer agent is the key to this photo-mediated reversible reaction. When the allyl-sulfide on the chain-transfer agent is introduced to a thiolated protein, it releases the old protein and regenerates the ‘ene’ functionality. Then it can be reacted with the new thiolated protein. And this procedure can be done multiple times.
Reversible bioconjugation using thiol allows researchers to overcome challenges that emerge in covalent bioconjugation. This same photo mediated technique also allows researchers to pattern proteins on hydrogels and release the protein via the photolabile linkage (source). Consider reading our article about hydrazine bioconjugation, which uses pH to reverse conjugation.
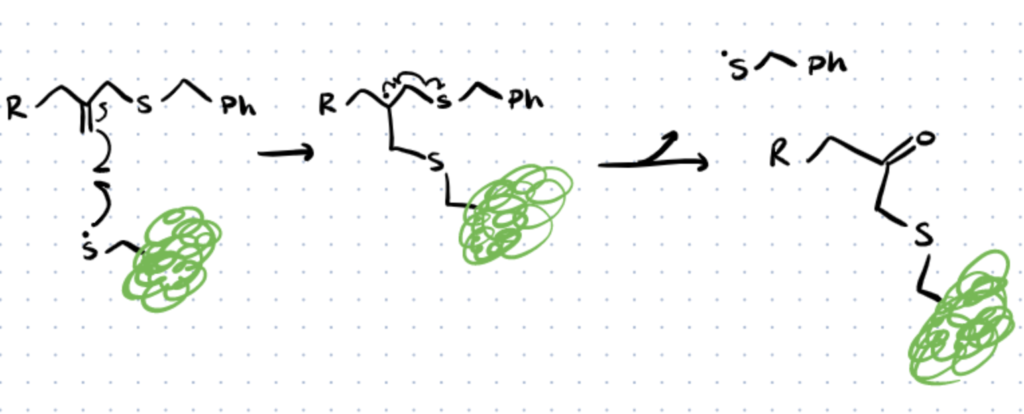
Other Thiol-Mediated Bioconjugation Techniques
Other thiol-mediated bioconjugation techniques include thiol-para fluoro, thiol-yne, thiol-vinyl sulfone, thiol–pyridyl disulfide, and thiol–bisulfone reactions.
In this section we’ll discuss several other thiol-mediated bioconjugation techniques which are sometimes used in literature. You can find more details in this source article.
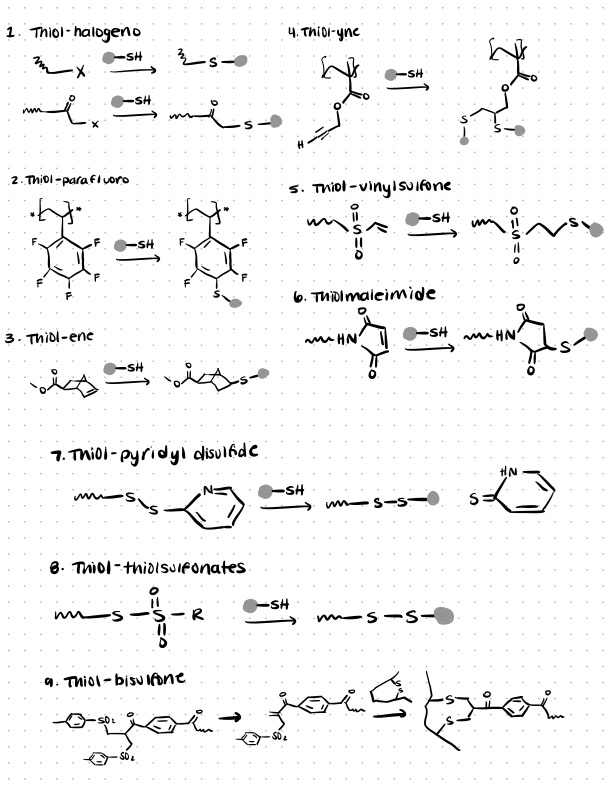
Thiol-parafluoro Reactions
Pentafluorostyrenes which are used for making poly-electrolyte membranes for fuel cells are polymerized using various techniques. Using thiol-parafluorothiols is advantageous because they can be used to create many different kinds of polymers and it is easy to observe reactions with thiols using 19F NMR spectroscopy. Thiol-parafluorothiol reactions are also quicker than other techniques however an excess of NEt3 is needed to produce complete conversion.
Thiol-ene Reactions
Thiol-ene reactions can be used for photo-joining of peptides. However, even though the thiol–ene reaction is referred to as click chemistry, by-products like dimers and disulfides have been noted during the course of the reaction. Also, because of lengthy reaction times in UV–vis light, only peptides that show stability in such an environment are suitable for thiol-ene reactions.
Thiol-yne Reaction
Thiol-yne reactions can be utilized for synthesizing glycopolymers. This chemistry is rarely utilized and there are only a few reports of its use in bioconjugation and synthesis of glycopolymers. One reason to use thiol-yne reactions instead of thiol-ene reactions is because it’s possible to react two thiols with one alkyne and produce polymers with dendritic carbohydrate-like capabilities. However, using large thiols hinders full conjugation because of stericity.
Thiol-vinyl Sulfone Reaction
Thiol-vinyl sulfone reactions are frequently used to create polymers via reversible addition fraction transfer polymerization. These reactions don’t need catalysts and full conversion can be reached in less than 24 hours by simply stirring the monomers in an organic solvent or in an aqueous solution that is slightly alkaline. Additionally, it’s easy to crease polymers with thiol functional groups via basic aminolysis.
Thiol-pyridyl Disulfide Reaction
The quick reaction between thiols and pyridyl disulfides is a preferred method for polymer–peptide conjugates. Assorted disulfides are the product of a quick exchange between the pyridyl disulfide with free thiol groups. Additionally, during the reaction of linkers like SPDP, a yellow pyridine-2-thione product makes it easy to observe the rate of the reaction. What makes this reaction different from the rest is that the linkage that joins the polymer and biomolecule is formed via a disulfide bridge, and can be cleaved once more which enables the biomolecule to be released from the reductive cell surrounding.
Thiol-bisulfone Reaction
The unusual thiol–bisulfone reaction can be used to insert a new linker into disulfide bridges (which are commonly found in proteins and certain cyclic peptides).
