Some of the most common targets of bioconjugation are proteins. Bioconjugated proteins and peptides have a wide range of biological and biomedical applications, including drug delivery, assays, and fluorescent visualization. When developing procedures to make protein bioconjugates, researchers can choose to target the proteins’ N-termini, C-termini, or amino acid side chains. In this overview, we will look specifically at N-terminal bioconjugation of proteins.
Methods for N-terminal bioconjugation of proteins include one-step reactions with NHS-esters, site-specific conjugation to engineered amino acids, enzymatic n-terminal bioconjugation, and two-step reactions using chemicals like pyridoxal-5-phosphate.
What is N-terminal Bioconjugation?
In bioconjugation, it’s important to find reactions and techniques that work on specific functional groups. One such target can be the N-terminus in proteins. There are multiple benefits of using a terminal site over a side chain for protein bioconjugation. Often the terminal sites exist in a unique, solvent-exposed chemical environment and there is only one terminal of each type per protein; these properties allow for more directed conjugation. Furthermore, conjugating other molecules onto the terminals usually doesn’t affect protein function. The N-terminal is used more frequently than the C-terminal since its 𝛼-amine is particularly reactive (Jiang et al., 2021; Rosen and Francis, 2017).
Related articles:
- Learn about choosing the right bifunctional crosslinkers to react with amine groups here.
N-terminal bioconjugation is a bioconjugation strategy that targets the 𝛼-amine at the N-terminus of proteins.
Why Undergo N-terminal Bioconjugation?
N-terminal bioconjugation of proteins can be used to attach probes, such as fluorophores, other proteins, or protecting groups like methyl groups which prevent degradation of proteins.
This allows proteins and peptides to be functionalized for specific uses. It’s important in the pharmaceutical industry, as it’s used to improve drug efficacy and delivery. It’s also commonly used in assays and bioimaging. Therefore it can be of interest to any researchers studying or using proteins or peptides.
Common probes attached to the N-terminus include biotin, polyhistidine tags, PEG, and fluorophores. Biotin is an important tool due to its affinity for the proteins avidin and streptavidin. Streptavidin-coated surfaces can be used to capture biotinylated proteins in assays. You can learn more about biotin-streptavidin conjugation techniques in our other article.
Polyhistidine tags are peptides with at least six consecutive histidine residues. The tags can bind to transition metal ions, such as Ni2+ or Co2+, which then bind to acids such as nitrilotriacetic acid (NTA) or iminodiacetic acid (IDA). NTA or IDA can be fixed to beads to isolate and purify the proteins (Sinobiological).
Fluorophores are a diverse group of molecules that emit fluorescence. They can be designed to be activated or quenched upon meeting the proper binding partner. This property of signal modulation is used in assays like FRET and PET (Terai and Nagano, 2013).
You can also utilized N-terminal bioconjugation of radiopharmaceuticals to develop drugs that are both therapeutic and diagnostic (since the radiolabels can be imaged using PET and SPECT).
Most proteins and antibodies have readily available amines and carboxyls. You can label proteins and antibodies with these conjugation kits to save time and improve consistency between experiments.
PEG, or polyethylene glycol, is used widely in the pharmaceutical industry. It can change the properties of a molecule such as its hydrophobicity and binding affinities. As a result, it can improve the effectiveness of drugs, often decreasing their immunogenicity and increasing their retention (Veronese and Mero, 2008). Methylation and acetylation are common post-translational modifications (PTMs) in native peptides. They are used as functional groups in drugs, as well, and can increase selectivity and stability, and decrease cytotoxicity; however, the effects vary depending upon the drug (Orwig and Dix, 2005; Sun and Fu 2018).
What Are Different N-terminal Bioconjugation Strategies?
There are a few considerations that need to be taken into account by researchers. Often, proteins from eukaryotic expression systems are acetylated and unsuitable for modification at the N-terminus. However, proteins like antibodies have their N-terminal signal peptide cleaved and are therefore free from acetylation or other post-translational modifications. Therefore, it’s best to use proteins from prokaryotic expression systems or secreted eukaryotic proteins (Rosen and Francis, 2017).
Many strategies also rely on pH, taking advantage of the N-terminus’ relatively low pKa and subsequent deprotonation and charge at neutral pHs. This property can be used to differentiate the N-terminus from other nucleophilic sites, such as the 𝜀-amine site found on lysine residues. The downside to these reactions is that they have selectivity issues and tend to create heterogeneous mixtures (Jiang et al., 2021; Rosen and Francis, 2017).
N-terminal Bioconjugation Using One-Step Reactions
The most basic N-terminal bioconjugation reactions target the terminus directly in one step. One example is N-hydroxysuccinimide, or NHS ester bioconjugation which enables you to attach a desired product to the N-terminal via an amide bond. This makes use of the N-terminus’ low pKa as previously discussed, however, this method still works best in proteins with few surface lysine residues to further prevent unwanted side reactions. We’ve covered site-specific lysine conjugation methods in our other article.
Other examples of direct methods use ketones and 2-pyrinecarbaldehyde (2-PCA) derivatives, which work in acylation and condensation reactions, respectively. Even catalyzed reactions can work by direct labeling — such as a reductive amination using benzaldehyde and a cyanoborohydride catalyst. There are many well-established methods in the literature with novel ones still being discovered. (De Rosa et al., 2021; Jiang et al., 2021; Rosen and Francis, 2017; Stephanopoulos and Francis, 2011).
Bioconjugation of Specific Amino Acids at the N-Terminus
One strategy to better target the N-terminus is to increase selectivity by using reactions that target specific side chains at the terminal; this makes these reactions not as universally applicable, however, unless proteins are engineered. The most common targets are cysteine, proline, and glycine, although serine, threonine, and tryptophan are occasional targets, as well.
N-terminal cysteines are often used because they rarely occur naturally. Native chemical ligation (NCL) is a cysteine-targeting technique that is used with a thioester probe and creates an amide bond. However, it can suffer from issues such as slow kinetics and a lack of selectivity, therefore techniques like nucleobase-involved NCL are better. Proline also allows for high selectivity because it contains a secondary amine instead of a primary amine. (De Rosa et al., 2021; Jiang et al., 2021; Stephanopoulos and Francis, 2011).
You can also utilize palladium catalyzed bioconjugation to react with cysteines that are introduced at the N-terminus of proteins.
Two-Step Reactions That Are Orthogonal to Lysine Bioconjugation
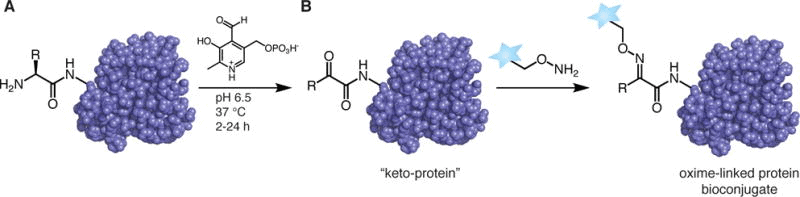
Some reactions occur in multiple steps, first changing the functional group at the N-terminal, then conjugating material to the new functional group. The amino group is most often converted to an aldehyde, but reactions that convert it to a ketone or azide are also used. If you’re curious about azide bioconjugation techniques, read more here.
One example is to use pyridoxal 5′-phosphate (PLP) to create an imine which will tautomerize into a ketone or aldehyde; this reaction avoids side-chain interactions with lysine. There are many options for the second reaction step, but one is to react with a newly formed N-terminal aldehyde with an alkoxyamine probe (De Rosa et al., 2021; Rosen and Francis, 2017).
Enzymatic N-Terminal Bioconjugation
Another option is to use enzymes. Common options are Sortase A, which acts on a specific peptide sequence called a sortag, N-Myristoyl transferase (NMT), which can add azido or alkyne-containing myristic acid analogs for use in CuAAC or SPAAC reactions, subtiligase, which has total chemoselectivity, and Butelase 1, which has yet to be expressed recombinantly and therefore has issues with its availability (De Rosa et al., 2021; Jiang et al., 2021; Rosen and Francis, 2017).
Applications of N-terminal Bioconjugation of Proteins
Some applications of N-terminal bioconjugation include the creation of biomedical implants, the creation of nanocarriers for drug delivery, and the expansion of the field of proteomics.
Application 1. Creation of a Platform for Dental Implants
Miller et al., (2020) ligated collagen fibers onto a titanium surface in a multi-step process. First, they added an amine functional group to the titanium, then they changed the functional group at the N-termini of the collagen to ketones with a chemical reagent called Rapoport’s salt, which then formed oxime bonds with amines on the titanium surface. The bound collagen proteins were exposed to a solution with free-floating collagen monomers so that they could undergo nucleation and grow.
This research provides the framework for a new method of creating dental implants that avoids issues with long-term stiffening and immobility; the resulting collagen fibrils perpendicular to the titanium can provide a platform onto which a synthetic periodontal ligament can be placed.
Application 2. Incorporation of Cargo into Viral Nanocages
Schoonen et al., (2018) used 2-pyridinecarboxaldehydes (2-PCAs) to add a bioorthogonal vinylboronic acid handle to the N-terminus of the cowpea chlorotic mottle virus (CCMV) capsid protein, which resides on the inside of the capsid. This handle can then be reacted with another complimentary handle that is bound to cargo, which can then be incorporated into the viral nanocage and used as a drug delivery mechanism. You can learn more about bioconjugation of nanoparticles and other nanocarriers in our related article.
Application 3. Discovering New Non-acetylated Proteins
Yoshihara et al., (2008) used subtiligase to acylate the N-termini of proteins with novel peptide esters containing biotin tags. The proteins were then digested with trypsin and bound to avidin using biotin-avidin interactions. At this stage, biotin molecules, the peptide portions of the original peptide ester, and the parts of the protein that contained the N-termini were bound together. TEV Protease then cleaved the peptides from the N-termini and the resulting N-termini were examined using LC-MS/MS. This technique is a means of discovering which proteins are not acetylated at their N-termini, as well as where their cleavage sites are. An alternative method might involve quantum dot bioconjugation for discovering these types of proteins.
A Method for N-terminal Methylation Using An Enzymatic Approach
In the following steps, we present a general method for N-terminal methylation based on a study where the researchers tested the activity of NRMT, a newly discovered methyltransferase. For more information, including other assays they performed and more complete protocols, you can look at this paper, published by Schaner Tooley et al., 2010.
Polymers can be conjugated to proteins and antibodies using a range of functional groups such as amines, carboxyls, or thiols. Explore conjugation kits for polymers, proteins, and antibodies, here.
Step 1. Express and Purify Proteins
Express N-terminal RCC1 methyltransferase (NRMT) and RCC1 proteins in a mammalian expression system and tag them with His6 tags and FLAG tags. Purify proteins using Ni-NTA beads.
Step 2. Methylate RCC1 and Assay
Elute Flag-NRMT into an MTase buffer, then add RCC1 and SAM (S-adenosyl methionine). Analyze results for methylation with an immunoblot assay. Additionally, add Flag-NRMT to calcium phosphate transfected cells. Mix nuclear extract with RCC1 and SAM and assay on ELISA-coated plates.
A Method for N-terminal Biotinylation
In the following steps, we present a general method for N-terminal biotinylation. It demonstrates the potential for enzymatic biotin tagging via the protein BirA as opposed to chemical biotinylation. More details can be found in this paper by Fairhead and Howarth, 2015.
Step 1. Produce GST-BirA Proteins
Transform an E. coli strain with the pGEX-GST-BirA plasmid. After ensuring that a colony will grow in ampicillin, grow to an OD600 of 0.5, then induce protein expression with IPTG (Isopropyl β-d-1-thiogalactopyranoside). When cells are ready to be lysed after harvesting, add a solution with lysozymes. After cells are digested and again harvested, add glutathione Hi-Cap resin to help purify and harvest the GST-BirA protein.
Step 2. Create AviTagged Proteins
Run PCR with KOD polymerase. Appropriate forward and reverse primers to create AviTag peptides can be found in the paper. After 12 amplification cycles, digest with the restriction enzyme DpnI. Add T4 DNA ligase and T4 polynucleotide kinase, then transform and overexpress the plasmid in E. coli.
Step 3. Biotinylate AviTagged Proteins
Add AviTagged proteins, magnesium chloride, ATP, GST-BirA, and D-Biotin to a PBS (phosphate-buffered saline) solution. Incubate and add fresh GST-BirA and Biotin. Collect and dialyze the sample.
Step 4. Test Biotin-Streptavidin Interactions
Boil and cool a solution of biotinylated proteins with SDS-PAGE buffer. Add streptavidin to the samples and run on an SDS-PAGE gel. When analyzing the gel, it is important to note that streptavidin has multiple binding sites and therefore can associate with 1 or 2 chains of the biotinylated target and may therefore create multiple bands.
A Method for N-terminal Acetylation
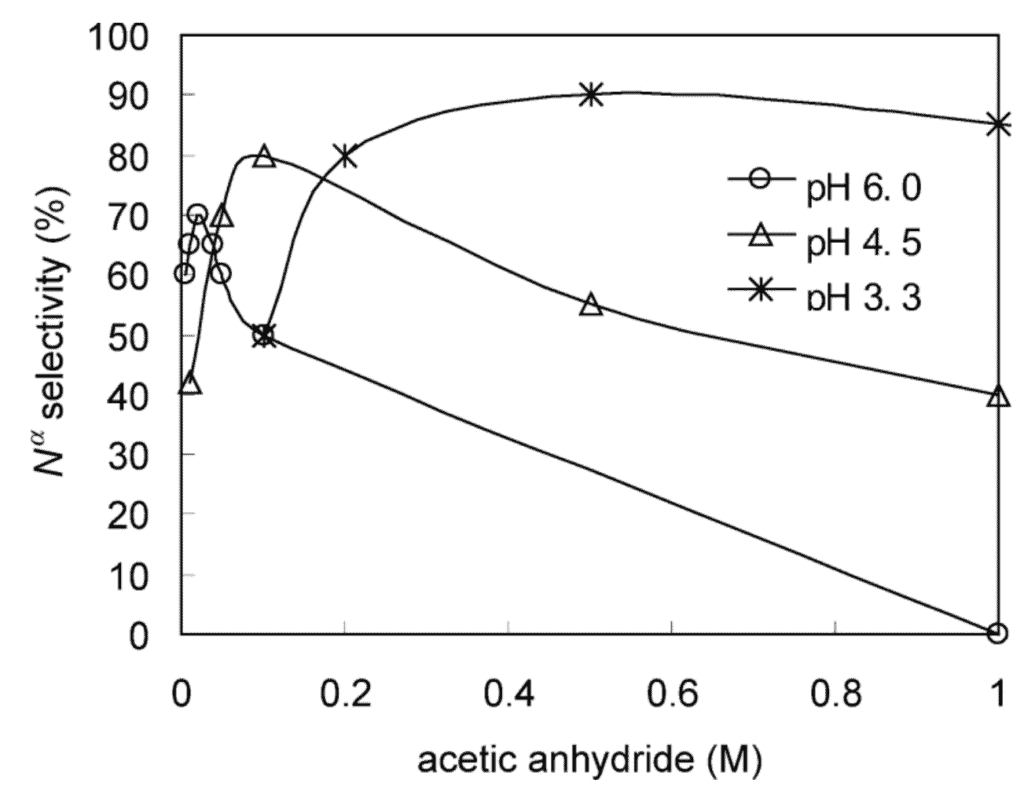
Here we present a general method for N-terminal acetylation. In this paper, Mikami et al., 2012, used acetic anhydride to selectively acetylate peptides.
Step 1. Acetylate Peptides
Add dynorphin A peptides to either a 0.1% acetic acid solution or a pyridine-acetic acid buffer. Incubate in an ice bath, then add acetic anhydride, either with anhydrous tetrahydrofolate or acetonitrile. Vortex the solution, incubate in an ice bath again, then dry with a SpeedVac.
Step 2. Analyze Peptides with Mass Spectrometry
Use fast atom bombardment mass spectrometry at an accelerating voltage of 10kV and a mass resolution of 1:1000 to confirm selective acetylation at the N-terminus.
Mass spectrometry can also provide information regarding the composition and size of macromolecules such as proteins, oligonucleotides, oligosaccharides, lipids, and even amino acids. For more information involving mass spectrometry applications related to bioconjugation, read our article on bioconjugation characterization.
A Method for N-terminal Labeling With a Fluorophore
In the following steps, we present a general method for N-terminal labeling with a fluorophore. It was used to study the effect of antibody-fluorophore bioconjugation. More details can be found in this paper by Szabo et al., 2018.
Step 1. Express and Purify Antibodies
Purify antibodies from the supernatant from a mouse hybridoma cell line using protein A affinity chromatography.
Step 2. Conjugate Fluorophores and Antibodies
Conjugate the NHS esters of AlexaFluor546 and AlexaFluor546 dyes with the antibodies according to the manufacturer’s instructions. The dye-to-protein ratio for each combination can vary between 0.5 and 6 and should be determined with spectrophotometry.
Step 3. Label Cells with Antibodies
Label cells in a PBS buffer that is supplemented with BSA (bovine serum albumin) and the conjugated antibodies.
Step 4. Test Fluorescence
Use a FACS Aria III flow cytometer to test fluorescence emissions. The AlexaFluor546 will be excited by a 561 nm laser beam and the AlexaFluor546 will be excited by a 633nm laser beam.
