Nucleophilicity and solvent-exposure are the most important properties to consider in protein bioconjugation targets. While some research has been conducted on amino acids with hydroxyl groups, such as serine and threonine, the bulk of the literature focuses on conjugating amine and thiol groups. Therefore, the N-terminus of a protein, cysteine residues, and lysine residues are the most common bioconjugation targets. In this overview, we will look at lysine conjugation strategies, protocols, and applications.
Lysine conjugation typically involves reaction with NHS-esters. Use heteroaryls, ꞵ-lactams, ꞵ-diketones, vinylsulfonamide, or cysteine-to-lysine transfers for site-specific lysine conjugation.
What is Lysine Conjugation?
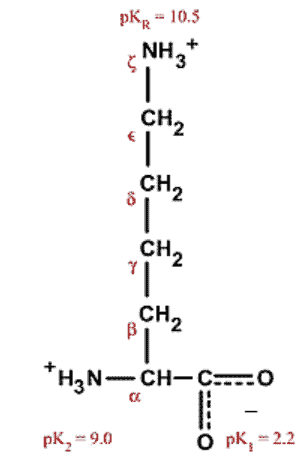
Lysine is an amino acid that contains a nucleophilic 𝜀-amine group which is frequently targeted by electrophilic linkers. A variety of different reagents are used, however acylation, alkylation, and arylation are the most common mechanisms.
Lysine conjugation is the conjugation of probes, fluorophores, or other small molecules such as drugs to the 𝜀-amine of the lysine side chain. It is often utilized in the production of antibody-drug conjugates (ADCs), protein visualization studies, and biochemical assays.
ADCs (antibody drug conjugates) are an especially exciting emerging area of biomedical and cancer research which allow for cytotoxic drugs to be transported within the body. Small cytotoxic drugs are conjugated to antibodies and delivered to cells of interest, at which point the linker is cleaved and the drug is released. Lysine bioconjugation is utilized for several ADCs that are under development. However, lysine conjugation has several disadvantages. Below we’ll discuss the pros and cons. If you’re looking for alternative methods for antibody bioconjugation, take a look at the linked article.
Antibodies can be easily attached to other biomolecules like proteins, polymers, and carbohydrates using amines, carboxyls, and even thiols. Use these antibody conjugation kits to attach antibodies with other biomolecules.
Pros and Cons of Lysine Conjugation
Since lysine is among the most common amino acids, any reagent that targets 𝜀-amines will conjugate multiple residues across the surface of the protein. This is helpful when signal amplification is beneficial, such as in fluorophores, but this can also have deleterious effects on protein function, including the pharmacokinetics of antibody-drug conjugates. For less commonly explored amino acids, explore our article about histidine bioconjugation.
Most reagents used are not lysine-specific. In general, the thiol group of cysteine and ⍺-amine of the N-terminus are more nucleophilic, so it is difficult to exclude them from conjugation reactions. However, there are some new avenues of research that are achieving both lysine-specificity and site-specificity. You can also, alternatively, just react with the cysteine groups via maleimide bioconjugation reactions.
It is also important to avoid buffers like Tris which contain free amines, as they will compete with the amines on the protein surface and reduce the efficacy of the conjugation.
Additionally, when you’re trying to make a defined biomolecule (for example an antibody drug conjugate) where you need to know and control the precise location of conjugation, lysine conjugation is challenging to use. This is because there are so many lysines on most protein surfaces that there is tremendous batch-to-batch variability in bioconjugation locations.
Lysine Bioconjugation Strategies
Lysine bioconjugation strategies include general conjugation to lysines using NHS-esters or maleimides, site-specific antibody conjugation using lysines, and cysteine-to-lysine transfers.
It is important to note that there are many more reagents and methods for lysine bioconjugation than we will cover here. We are simply focusing on some of the more common, novel, and interesting strategies.
NHS-Lysine Conjugation
NHS (N-Hydroxysuccinimide) esters are among the most common linkers used, as they create stable amide bonds with amines through an acylation reaction. However, they present several challenges. They react not only with both ⍺- and 𝜀-amines, but also with thiols and hydroxyl groups in other amino acids. NHS-esters also hydrolyze rapidly in water. This can be remedied by increasing the amount of the target protein in the buffer, using an organic solvent, or using sulfonated analogs.
We’ve discussed NHS ester bioconjugation techniques in detail in another article. You can learn more about hydrolysis and other challenges with bioconjugation in water in our related article.
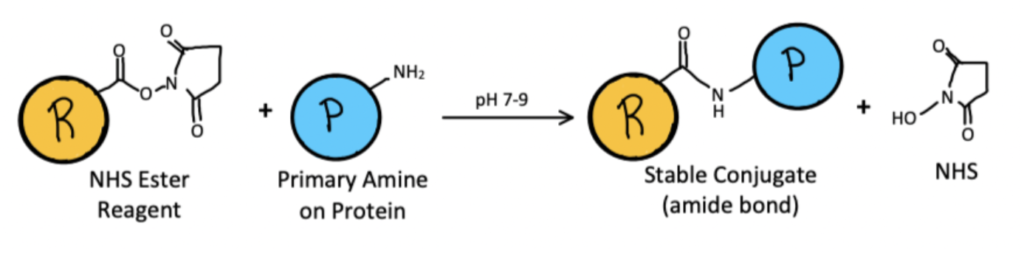
A Method for Lysine Conjugation Using NHS Esters
Here we present a bioconjugation method from a paper that used NHS ester bioconjugation, as well as the click reaction, to find new druggable hotspots in the proteome. For the full protocols, read this article.
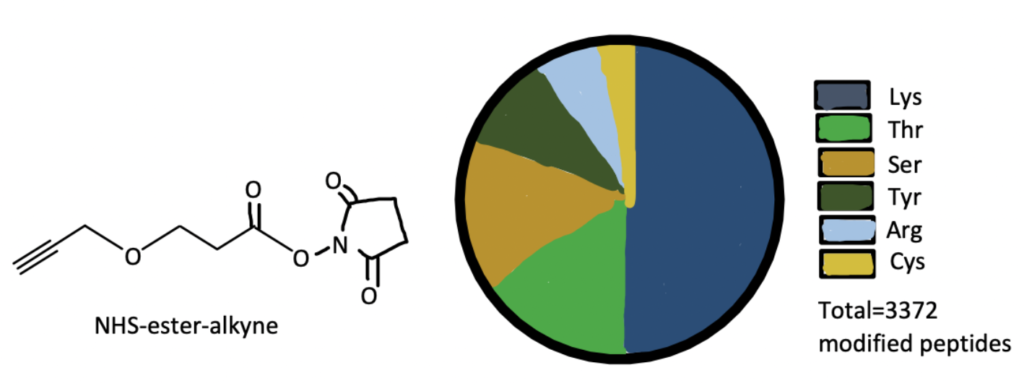
Step 1. Synthesize Dichlorotriazine Alkyne Probe
Add propargyl amine to an ice-cold solution containing cyanuric chloride, diisopropylethyl amine, and tetrahydrofolate, then stir for 3 hours. Filter the mixture and remove the solvent with a vacuum, then purify the residue with flash chromatography to yield 3,5-dichloro-N-(prop-2-yn-1-yl)-aniline. Biotinylate at room temperature.
Step 2. Prepare Proteome Samples
Harvest mouse liver tissue, then flash freeze in liquid nitrogen. Homogenize in PBS (phosphate-buffered saline), then centrifuge at 100,000g for 45 minutes to remove debris.
Step 3. Bioconjugate Lysines with NHS-Ester
Dilute liver samples in PBS to 2mg/mL. Add 2,5-dioxopyrrolidin-1-yl-3-(prop-2-ynyloxy)propanoate, a commercially available NHS-ester, and let incubate for an hour at room temperature. This will conjugate the NHS-ester to the lysine residues in the proteome samples.
Step 4. Perform the Click Reaction
In sequential order, add tris(2-carboxyethyl)phosphine, tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine, copper(II) sulfate, and the biotin-linked alkyne probe synthesized in step 1.
Step 5. Analyze Results
Use isoTOP-ABPP methods to link the proteins to streptavidin beads, then digest with trypsin and analyze with liquid chromatography mass spectrometry.
Site-specific Antibody Conjugation Using Lysines
Some reagents, such as isothiocyanates, sulfonyl chlorides, and imidoesters are better than NHS esters at specifically targeting primary amines. However, they tend to target N-termini and lysine side-chains alike. New methods are being discovered that exclusively target lysine residues. For example, N-Methyl-N-phenylethenesulfonamide is a vinylsulfonamide which has been found to selectively modify lysine side-chains even in the presence of unmodified N-termini and enzymes, such as sortase A and microbial transglutaminase, have also been used to achieve lysine-specific bioconjugation. You can find more protocols for enzymatic bioconjugation techniques in our other article.
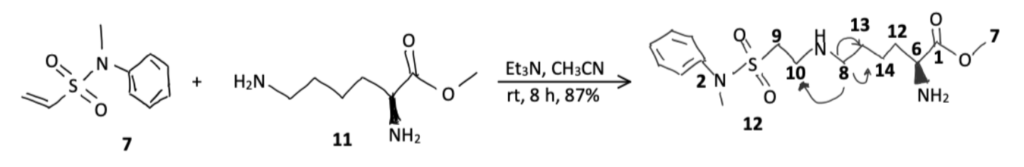
Another strategy is to target lysine residues that reside in hydrophobic pockets, as they are deprotonated at physiological pH and thus more nucleophilic than usual. This property can be leveraged to achieve not only lysine-specific conjugations, but site-specific conjugations. Reagents such as heteroaryls, ꞵ-lactams, and ꞵ-diketones have been used. These strategies are currently being used in the creation of ADCs.
A Method for Site-Specific Antibody Conjugation Using Lysines
Here we present a bioconjugation method from a paper that utilized the special properties of lysine residues in hydrophobic pockets to achieve site-specific antibody conjugation. For the full protocol, please read this paper.
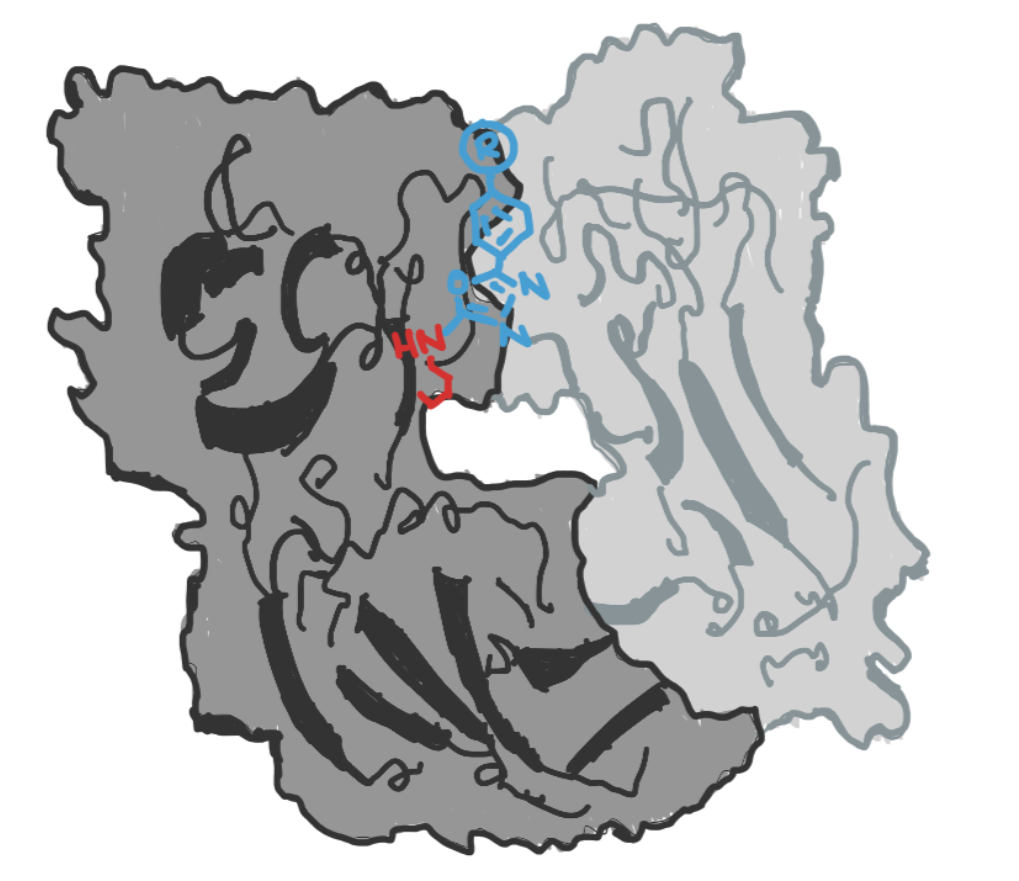
Step 1. Synthesize Fluorescein Derivative
The protocols for this step can be found in a separate paper here. Oxidize methylthioethers with mCPBA (meta-Chloroperoxybenzoic acid) to yield methylsulfones, then reduce azide groups via hydrogenation. Lastly, couple the compounds with NHS-5(6)-fluorescein to yield the methylsulfone fluorescein derivative.
Step 2. Purify Antibodies
To purify h38C2 IgG1 antibodies, clone the encoding sequencing into the mammalian expression vector pCEP4. Transfect an embryonic human kidney cell line and purify the supernatant with affinity chromatography after 5 days.
Step 3. Conjugate the Antibodies with the Fluorescein Derivative
Incubate h38C2 IgG1 antibodies with the methylsulfone fluorescein derivative at a 1:10 ratio in PBS (phosphate-buffered saline) for 3 hours at room temperature. Visualize results with SDS-PAGE Coomassie Blue gel staining. The methylsulfone fluorescein derivative will react site-selectively with lysines in the hydrophobic pocket. If you are interested in learning about other site-selective ways to attach to antibodies, consider our article on C-terminal bioconjugation methods and our article on transglutaminase bioconjugation, which is selective for a specific peptide sequence.
Maleimide Lysine Conjugation
Maleimides are a type of Michael acceptor and therefore undergo addition reactions with thiols. For this reason, maleimides are most often used as reagents for cysteine bioconjugation in literature. Between pHs of 6.5 and 7.5, they are highly selective for thiol groups. However, at more basic pHs, the reaction tends to slightly favor primary amines and a heterogenous mixture of modified thiols and amines is produced. For more information on cysteine bioconjugation techniques, read our article.
Thiols react readily and can be used for conjugation reactions. Papyrus Bio has a range of thiol-based conjugation kits. Explore thiol conjugation kits here.
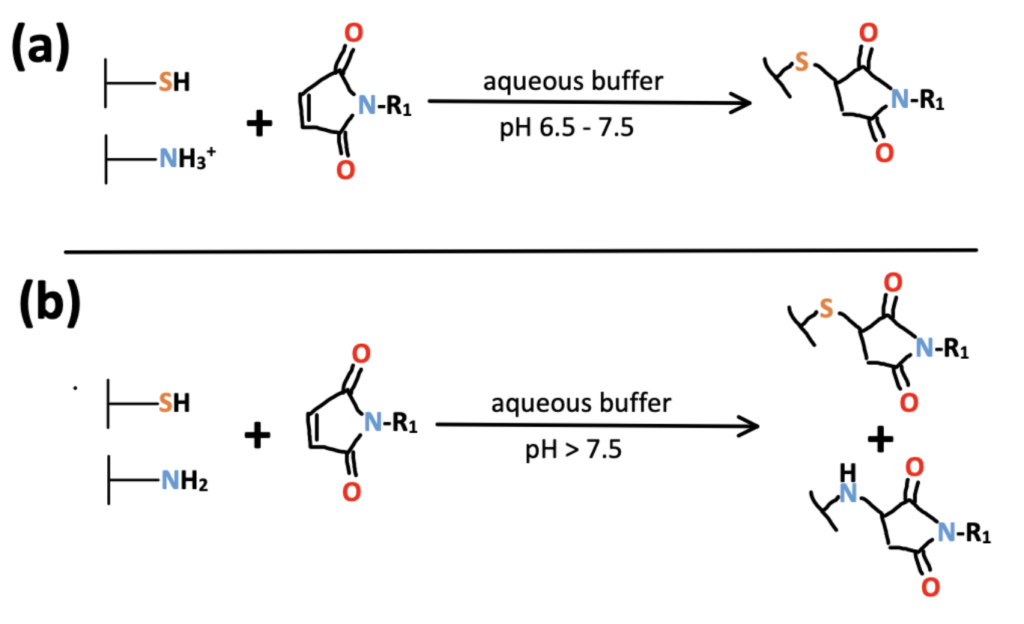
As with NHS-esters, maleimides are common reagents with many commercially available derivatives. If your protein contains a lot of cysteines, maleimides will enable you to conjugate to many of the cysteines – this is particularly useful for signal amplification and imagine purposes.
Cysteine-To-Lysine Transfer Antibody Fragment Conjugation
Researchers recently discovered a novel method of lysine conjugation called Cysteine-to-Lysine Transfer (CLT). Acylating reagents “hook” onto cysteine residues, then subsequently modify neighboring lysines. This is being used to achieve site-specific lysine conjugation at regions of antibodies which won’t interfere with the antigen binding sites.
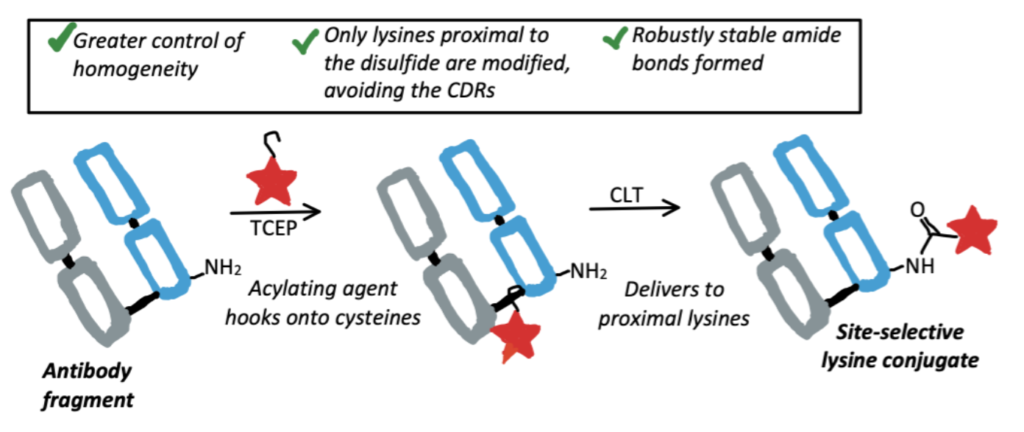
A Method for Cysteine-to-Lysine Transfer
Here we present a bioconjugation method from a paper that utilized CLT to selectively modify antibodies. For the full protocols, please read this paper.
Step 1. Synthesize the MESNa Thioester
Add sodium 2-(pent-4-ynoylthio)ethane-1-sulfonate and 4-pentynoic acid to an acetonitrile and dimethylformamide solution, then add N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline and stir at room temperature for 30 minutes. Then, add sodium 2- mercaptoethanesulfonate and stir at 80°C for 18 hours. Purify the solvent with affinity chromatography.
Step 2. Prepare Antibodies
Prepare the Trastuzumab Fab arm with a sequential enzymatic digest of the antibody with pepsin and papain.
Step 3. Perform Cysteine-to-Lysine Transfer
Add prepared antibody fragments to a conjugation buffer of phosphates, NaCl, and EDTA (ethylenediaminetetraacetic), then add TCEP (tris(2-carboxyethyl)phosphine). Incubate for 1 hour at 37°C. Add the MESNa thioester and incubate at 22°C for 4 hours. Exchange into BBS (borate-buffered saline), adjust the concentration to 20 μM, then incubate at 12°C for 72 hours. Analyze with LC-MS (liquid chromatography-mass spectrometry).
Lysine Conjugation Applications
Some applications for lysine bioconjugation include studying the efficacy of antibody-drug conjugates and using fluorophores to elucidate cellular pathways.
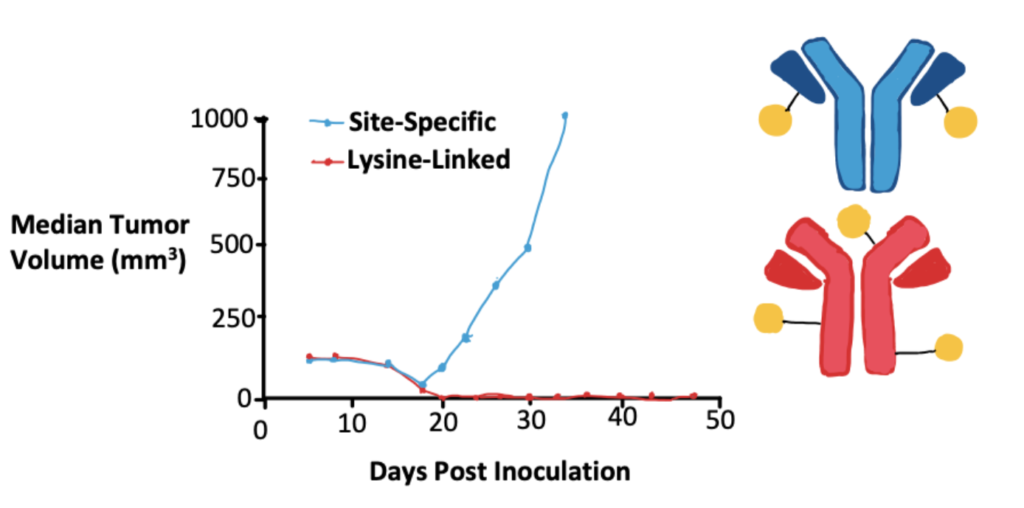
Application 1. Lysine-Conjugated ADCs
Yoder (2019) et al. compared ADCs that had heterogenous mixtures of lysine conjugates with ADCs that had site-specific cysteine conjugates. This study was performed in vivo to draw more meaningful, long-term conclusions and found that in this particular scenario the lysine-conjugated ADC was more effective. They draw attention to the necessity of in vivo testing to determine the efficacy of any particular variable, but due to the complexities of ADCs, can’t extrapolate their results beyond the scope of the variables studied.
Application 2. Protein Labeling with Fluorophores
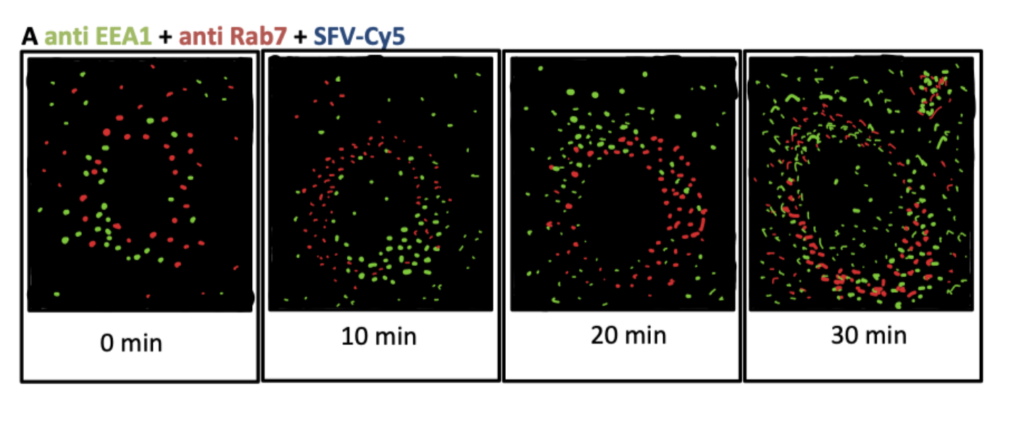
Vonderheit and Helenius (2005) labeled Semliki forest virus (SFV) particles with Cy5 NHS-ester fluorophores. SFV is internalized with clathrin mediated endocytosis, so they compared the locations of the fluorescent SFV particles with fluorescently labeled endocytic proteins to help visualize endocytic pathways. Their results indicate that the Rab7 protein is associated with early endosomes and facilitates both cargo sorting and transport vesicle formation. Similar to attachment of fluorophores, you might want to explore bioconjugation methods for radiopharmaceutical chemistry for visualizing proteins.